An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacists’ Perspectives Survey
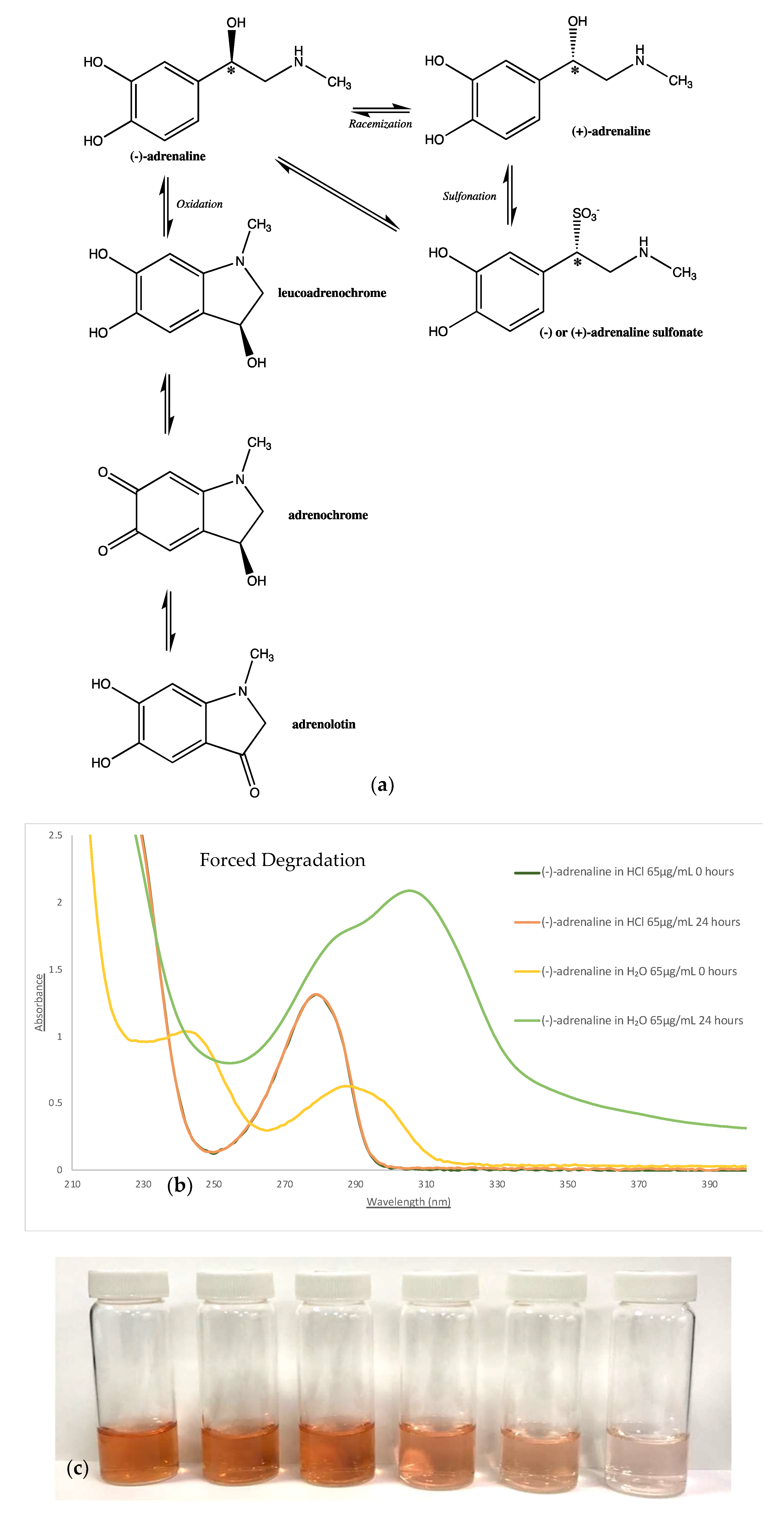
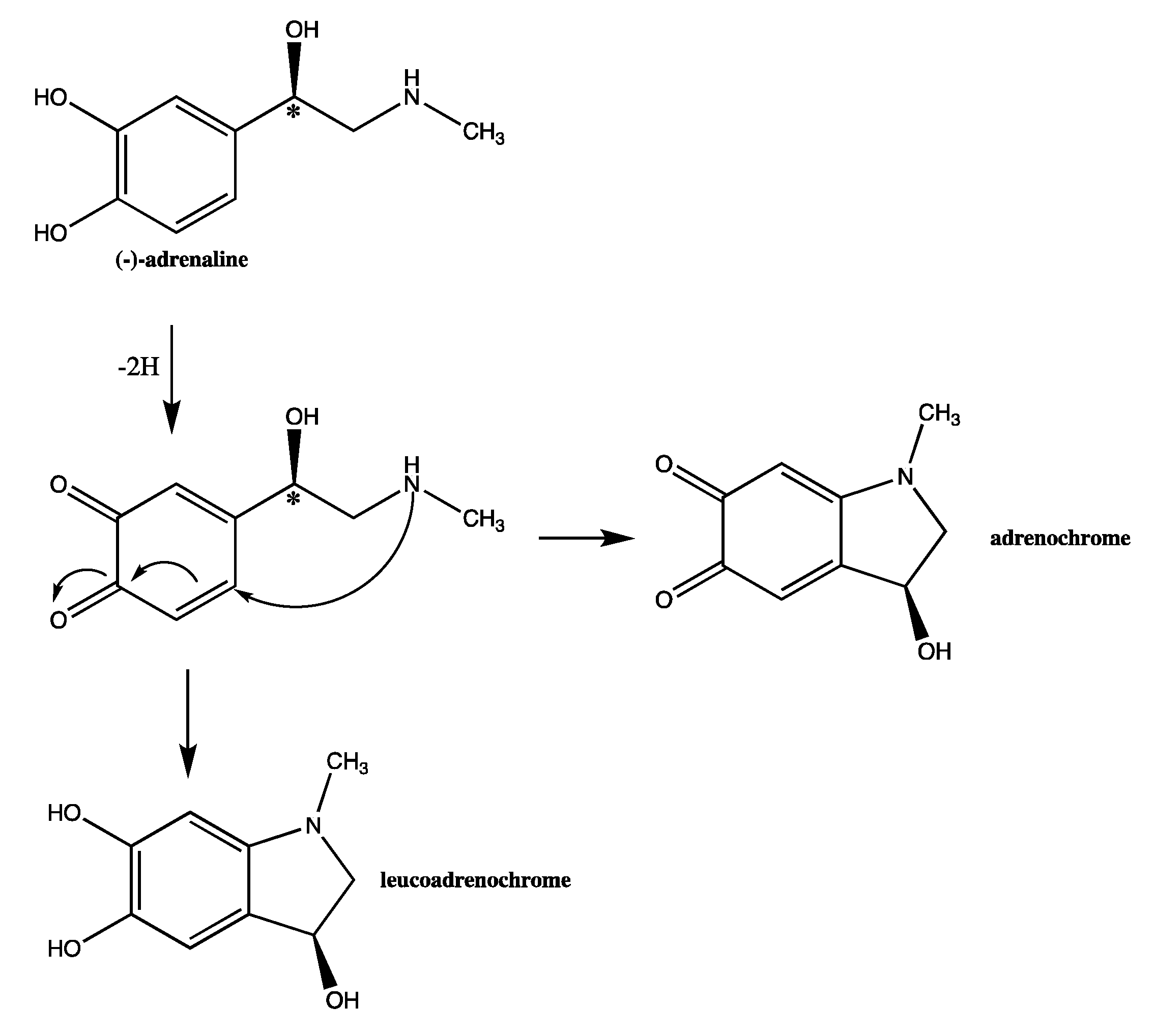
2.2. Exploration of Stability Forced Degradation
2.3. Preparation of Buffer and Model Formulations
2.4. Stress Tests
2.4.1. Vibration
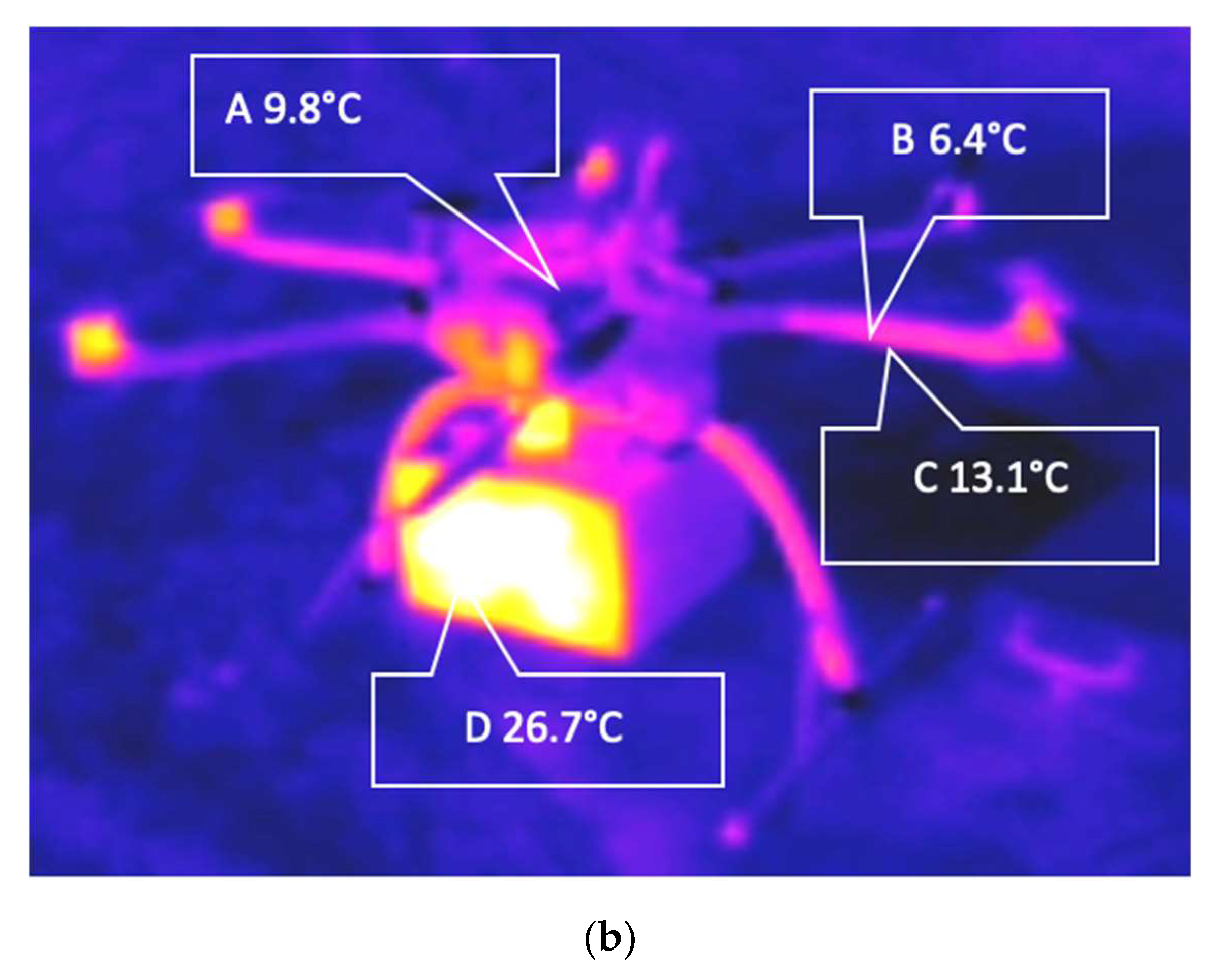
2.4.2. Temperature
2.4.3. Circular Dichroism Spectroscopy
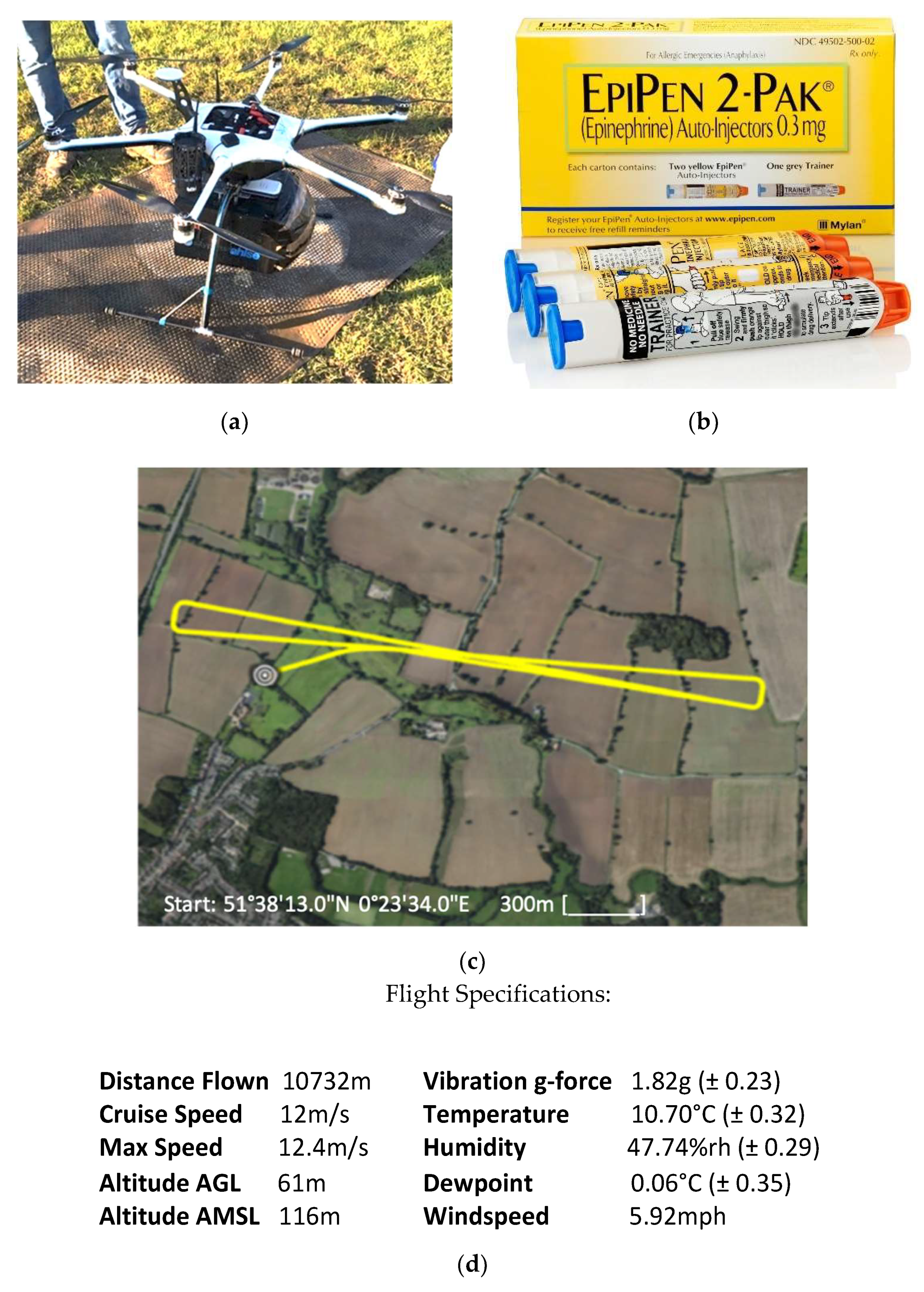
2.5. Drone Flight
2.6. Statistical Analysis
3. Results and Discussion
3.1. Stability Investigation
3.2. Stress Tests
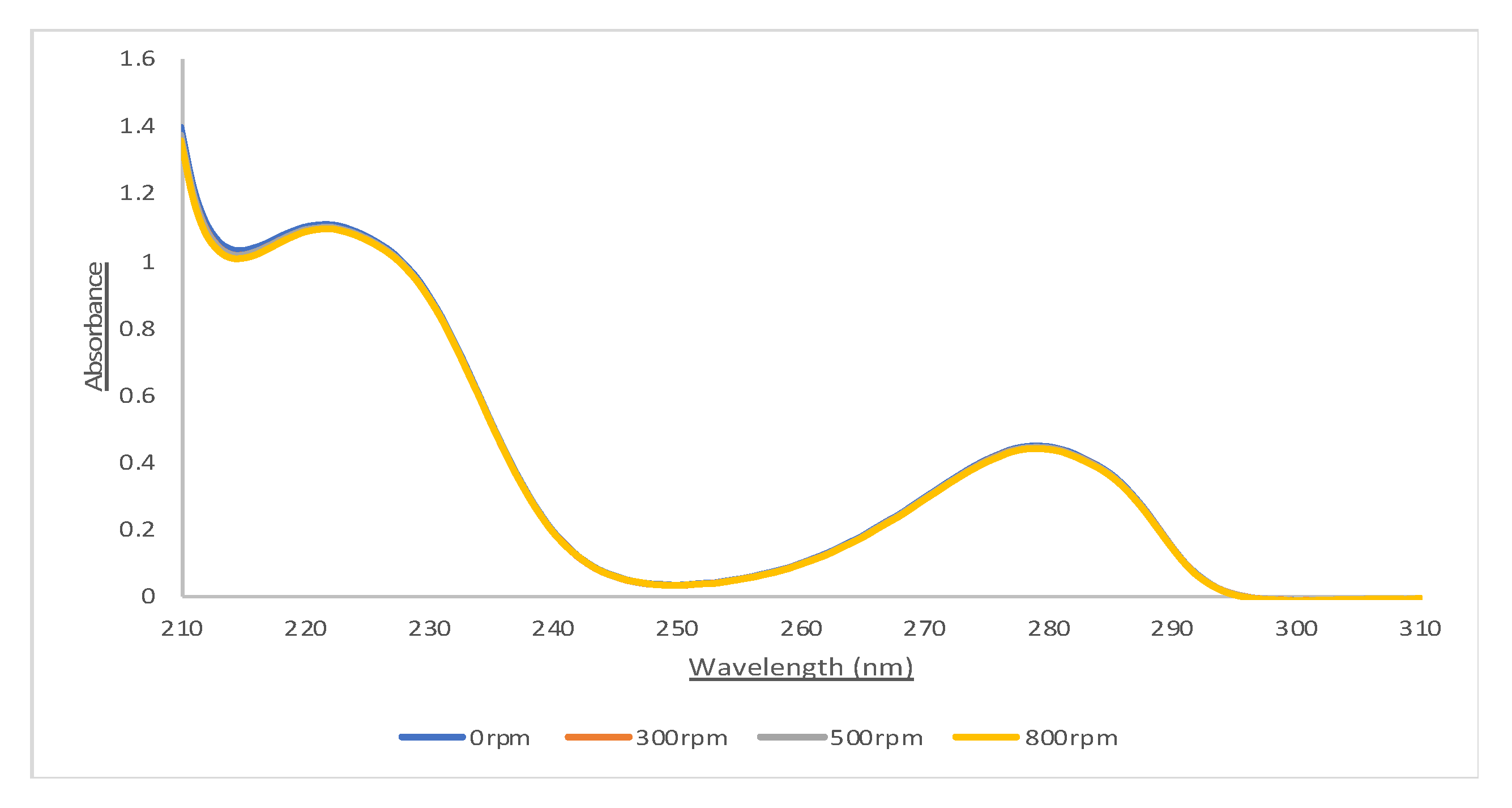
3.2.1. Effects of Vibration
3.2.2. Effects of Temperature
3.3. Drone Flight
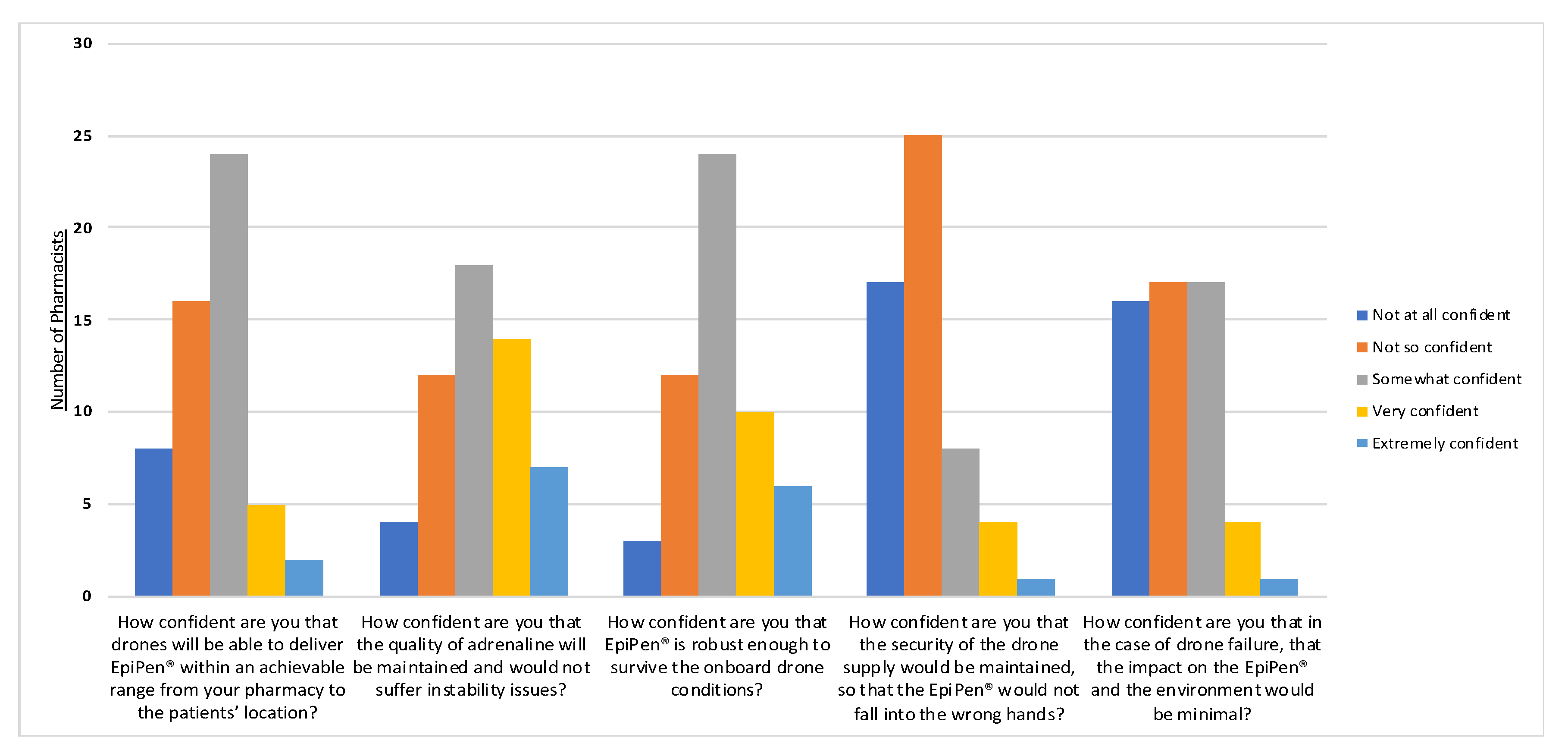
3.4. Pharmacists’ Perspectives Survey
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braun, J.; Gertz, S.D.; Furer, A.; Bader, T.; Frenkel, H.; Chen, J.; Glassberg, E.; Nachman, D. The promising future of drones in prehospital medical care and its application to battlefield medicine. J. Trauma Acute Care Surg. 2019, 87, S28–S34. [Google Scholar] [CrossRef]
- Phillips, A.; Sherwood, D.; Greenberg, N.; Jones, N. Occupational stress in Remotely Piloted Aircraft System operators. Occup. Med. 2019, 69, 244–250. [Google Scholar] [CrossRef]
- Mademlis, I.; Mygdalis, V.; Nikolaidis, N.; Montagnuolo, M.; Negro, F.; Messina, A.; Pitas, I. High-level multiple-uav cinematography tools for covering outdoor events. IEEE Trans. Broadcasting 2019, 65, 627–635. [Google Scholar] [CrossRef]
- Karaca, Y.; Cicek, M.; Tatli, O.; Sahin, A.; Pasli, S.; Beser, M.F.; Turedi, S. The potential use of unmanned aircraft systems (drones) in mountain search and rescue operations. Am. J. Emerg. Med. 2018, 36, 583–588. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A review on the use of unmanned aerial vehicles and imaging sensors for monitoring and assessing plant stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef]
- Rosser, J.C., Jr.; Vignesh, V.; Terwilliger, B.A.; Parker, B.C. Surgical and medical applications of drones: A comprehensive review. J. Society Laparoendosc. Surg. 2018, 22, e00018. [Google Scholar] [CrossRef]
- Balasingam, M. Drones in medicine—The rise of the machines. Int. J. Clin. Pract. 2017, 71, e12989. [Google Scholar] [CrossRef]
- Peckham, R.; Sinha, R. Anarchitectures of health: Futures for the biomedical drone. Glob. Public Health 2019, 14, 1204–1219. [Google Scholar] [CrossRef]
- Scarcella, J. Using Drones: What You Need to Know. Tech. Dir. 2016, 76, 25. [Google Scholar]
- Donnelly, L. The Telegraph, NHS Could Deliver Blood and Chemo Kits by Drone. Available online: https://www.telegraph.co.uk/news/2020/01/03/nhs-could-deliver-blood-chemo-kits-drone/ (accessed on 3 January 2020).
- Scalea, J.R.; Restaino, S.; Scassero, M. The final frontier? Exploring organ transportation by drone. Am. J. Transplant. 2019, 19, 962–964. [Google Scholar] [CrossRef]
- Scott, J.; Scott, C. Drone delivery models for healthcare. In Proceedings of the 50th Hawaii International Conference on System Sciences, Honolulu, HI, USA, 21 January 2017. [Google Scholar]
- Zegre-Hemsey, J.K.; Bogle, B.; Cunningham, C.J.; Snyder, K.; Rosamond, W. Delivery of Automated External Defibrillators (AED) by Drones: Implications for Emergency Cardiac Care. Curr. Cardiovasc. Risk Rep. 2018, 12, 25. [Google Scholar] [CrossRef]
- Nesta. Flying High: The Future of Drone Technology in UK Cities. Available online: https://media.nesta.org.uk/documents/Flying-High-full-report-and-appendices.pdf (accessed on 2 January 2020).
- Haidari, L.A.; Brown, S.T.; Ferguson, M.; Bancroft, E.; Spiker, M.; Wilcox, A.; Ambikapathi, R.; Sampath, V.; Connor, D.L.; Lee, B.Y. The economic and operational value of using drones to transport vaccines. Vaccine 2016, 34, 4062–4067. [Google Scholar] [CrossRef]
- Forster, K.; Turner, N. Revealed: NHS Ambulances Fail to Reach Most Seriously Ill and Injured Patients in Time Despite Efficiency Drive, The Independent. Available online: https://www.independent.co.uk/news/health/nhs-ambulance-response-time-targets-seriously-ill-injured-patients-efficiency-drive-secret-plans-a7690476.html (accessed on 20 December 2019).
- Anagnostou, K.; Turner, P.J. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch. Dis. Child. 2019, 104, 83–90. [Google Scholar] [CrossRef]
- Fleming, J.T.; Clark, S.; Camargo, C.A., Jr.; Rudders, S.A. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J. Allergy Clin. Immunol. Pract. 2015, 3, 57–62. [Google Scholar] [CrossRef]
- Kerddonfak, S.; Manuyakorn, W.; Kamchaisatian, W.; Sasisakulporn, C.; Teawsomboonkit, W.; Benjaponpitak, S. The stability and sterility of epinephrine prefilled syringe. Asian Pac. J. Allergy Immunol. 2010, 28, 53. [Google Scholar]
- PA Media. Number of Children Hospitalised in England with Severe Allergic Reactions Rises 72%, The Guardian, Allergies. Available online: https://www.theguardian.com/society/2019/nov/15/children-hospitalised-severe-allergic-reactions (accessed on 28 December 2019).
- Parish, H.G.; Morton, J.R.; Brown, J.C. A systematic review of epinephrine stability and sterility with storage in a syringe. Allergy Asthma Clin. Immunol. 2019, 15, 7. [Google Scholar] [CrossRef]
- Kraft, M.; Knop, M.P.; Renaudin, J.M.; Scherer Hofmeier, K.; Pfohler, C.; Bilo, M.B.; Lang, R.; Treudler, R.; Wagner, N.; Spindler, T.; et al. Secondary prevention measures in anaphylaxis patients: Data from the anaphylaxis registry. Allergy 2019, 75, 901–910. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Chan, E.S.; Gu, X.; Simons, K.J. Epinephrine for the out-of-hospital (first-aid) treatment of anaphylaxis in infants: Is the ampule/syringe/needle method practical? J. Allergy Clin. Immunol. 2001, 108, 1040–1044. [Google Scholar] [CrossRef]
- Brown, J.; Clowes, G. All About Epinephrine: What It Does in a Reaction, How Long It Lasts, When It Gets Hot or Cold, Allergic Living. Available online: https://www.allergicliving.com/2020/01/09/all-about-epinephrine-what-it-does-in-a-reaction-how-long-it-lasts-when-it-gets-hot-or-cold/ (accessed on 5 June 2020).
- Meda Pharma GmbH. This site is intended for UK Healthcare Professionals. Available online: http://www.epipen.co.uk/hcp/anaphylaxis-treatment/ (accessed on 2 January 2020).
- Rachid, O.; Simons, F.E.R.; Rawas-Qalaji, M.; Lewis, S.; Simons, K.J. Epinephrine doses delivered from auto-injectors stored at excessively high temperatures. Drug Dev. Ind. Pharm. 2016, 42, 131–135. [Google Scholar] [CrossRef]
- Church, W.H.; Hu, S.S.; Henry, A.J. Thermal degradation of injectable epinephrine. Am. J. Emerg. Med. 1994, 12, 306–309. [Google Scholar] [CrossRef]
- Grant, T.A.; Carroll, R.G.; Church, W.H.; Henry, A.; Prasad, N.H.; Abdel-Rahman, A.A.; Allison, E.J., Jr. Environmental temperature variations cause degradations in epinephrine concentration and biological activity. Am. J. Emerg. Med. 1994, 12, 319–322. [Google Scholar] [CrossRef]
- Lacwik, P.; Bialas, A.J.; Wielanek, M.; Sklodowska, M.; Kupczyk, M.; Gorski, P.; Kuna, P. Single, short-time exposure to heat in a car during sunny day can decrease epinephrine concentration in autoinjectors: A real-life pilot study. J. Allergy Clin. Immunol. Pract. 2019, 7, 1362–1364. [Google Scholar] [CrossRef]
- Pepper, A.N.; Westermann-Clark, E.; Lockey, R.F. The high cost of epinephrine autoinjectors and possible alternatives. J. Allergy Clin. Immunol. Pract. 2017, 5, 665–668. [Google Scholar] [CrossRef]
- Robinson, J. A Third of Community Pharmacies Cannot Order Supplies of EpiPen, Despite Distributor, saying They Are in Stock. The Pharmaceutical Journal. Available online: https://www.pharmaceutical-journal.com/news-and-analysis/news/a-third-of-community-pharmacies-cannot-order-supplies-of-epipen-despite-distributor-saying-they-are-in-stock/20207162.article (accessed on 20 November 2019).
- Stepensky, D.; Chorny, M.; Dabour, Z.; Schumacher, I. Long-term stability study of L-adrenaline injections: Kinetics of sulfonation and racemization pathways of drug degradation. J. Pharm. Sci. 2004, 93, 969–980. [Google Scholar] [CrossRef]
- Kongkiatpaiboon, S.; Duangdee, N.; Chewchinda, S.; Poachanukoon, O.; Amnuaypattanapon, K. Development and validation of stability indicating HPLC method for determination of adrenaline tartrate. J. King Saud Univ. Sci. 2019, 31, 48–51. [Google Scholar] [CrossRef]
- Rachid, O.; Simons, F.E.R.; Rawas-Qalaji, M.; Lewis, S.; Simons, K.J. Epinephrine autoinjectors: Does freezing or refrigeration affect epinephrine dose delivery and enantiomeric purity? J. Allergy Clin. Immunol. Pract. 2015, 3, 294–296. [Google Scholar] [CrossRef]
- Kirkpatrick, D.; Yang, J.; Trehy, M. Determination of the enantiomeric purity of epinephrine by HPLC with circular dichroism detection. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 556–563. [Google Scholar] [CrossRef]
- British Pharmacopeia Commission. Formulated Preparations: Specific Monographs–Adrenaline Injection/Epinephrine Injection; Adrenaline Solution/Epinephrine Solution; Adrenaline Acid Tartrate; British Pharmacopeia: London, UK, 2019. [Google Scholar]
- Schwirtz, A.; Seeger, H. Comparison of the robustness and functionality of three adrenaline auto-injectors. J. Asthma Allergy 2012, 5, 39. [Google Scholar] [CrossRef]
- ICH. Stability Testing of New Drug Substances and Products Q1A (R2), International Conference on Harmonization, IFPMA, Geneva. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 10 November 2019).
- National Center for Biotechnology Information. PubChem Database. Epinephrine, CID=5816. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Epinephrine. (accessed on 10 November 2019).
- Hii, M.S.Y.; Courtney, P.; Royall, P.G. An Evaluation of the Delivery of Medicines Using Drones. Drones 2019, 3, 52. [Google Scholar] [CrossRef]
- Deters, R.W.; Kleinke, S.; Selig, M.S. Static testing of propulsion elements for small multirotor unmanned aerial vehicles. In Proceedings of the 35th AIAA Applied Aerodynamics Conference, Denver, CO, USA, 5–7 June 2017; p. 3743. [Google Scholar]
- Kuantama, E.; Moldovan, O.G.; Ţarca, I.; Vesselenyi, T.; Ţarcă, R. Analysis of quadcopter propeller vibration based on laser vibrometer. J. Low Freq. Noise Vib. Act. Control 2019. [Google Scholar] [CrossRef]
- Mylan Inc. EPIPEN®* (Epinephrine Injection) Auto-Injector 0.3 mg. Available online: https://www.mylan.com/en/products/product-catalog/product-profile-page?id=6749373c-9fb4-4e2b-a856-3771b31f68f3 (accessed on 2 January 2020).
- Guzov, E.A.; Kazin, V.N.; Moshareva, V.A.; Zhukova, A.A. Application of Electronic Spectroscopy and Quantum-Chemical Modeling for Analysis of Products of Autoxidation of Adrenaline. J. Appl. Spectrosc. 2019, 85, 1107–1113. [Google Scholar] [CrossRef]
- Hoellein, L.; Holzgrabe, U. Ficts and facts of epinephrine and norepinephrine stability in injectable solutions. Int. J. Pharm. 2012, 434, 468. [Google Scholar] [CrossRef]
- Brustugun, J.; Kristensen, S.; Hjorth Tønnesen, H. Photostability of epinephrine–the influence of bisulfite and degradation products. Die Pharm. An. Int. J. Pharm. Sci. 2004, 59, 457–463. [Google Scholar]
- Beasley, H.; Ng, P.; Wheeler, A.; Smith, W.R.; McIntosh, S.E. The impact of freeze-thaw cycles on epinephrine. Wilderness Environ. Med. 2015, 26, 514–519. [Google Scholar] [CrossRef]
- ClogWorks Technologies Ltd. Available online: https://www.clogworks.com/DarkMatterhX (accessed on 5 May 2020).
- The Pharmaceutical Journal Online. Majority of Community Pharmacies Failed to Meet FMD Deadline, Figures Confirm. The Pharmaceutical Journal. Available online: https://www.pharmaceutical-journal.com/news-and-analysis/news-in-brief/majority-of-community-pharmacies-failed-to-meet-fmd-deadline-figures-confirm/20206183.article (accessed on 5 May 2020).
- Zilker, M.; Sorgel, F.; Holzgrabe, U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef]


| Critical Material Attribute | Parameter | Measurement and Method | ||
| Chiral Composition and Purity | Circular Dichroism | |||
| Enantiomeric Concentration | UV-Vis Spectroscopy | |||
| Visual Appearance | Visual Inspection | |||
| Critical Process Parameter | Parameter | Conditions | Time Course | Test Scenario |
| Temperature | 4–65 °C | 0, 0.5, 3, 24 h | Lab Stress Test | |
| Vibration | 0–13.33 HZ | 0, 0.5, 3, 24 h | Lab Stress Test | |
| Natural and UV Light | Natural light and UV | 0, 0.5, 3, 24 h | Lab Stress Test | |
| Duration, Pressure, and %Relative Humidity | Ambient | 18 min | Drone Flight | |
| British Pharmacopeia Criteria: | |
|---|---|
| Colour and Clarity Test | Physical stability of all solutions was determined by visual inspection against a black and/or white background using standard laboratory lighting. Solutions were examined for signs of colour change, precipitate formation, or particulate matter. |
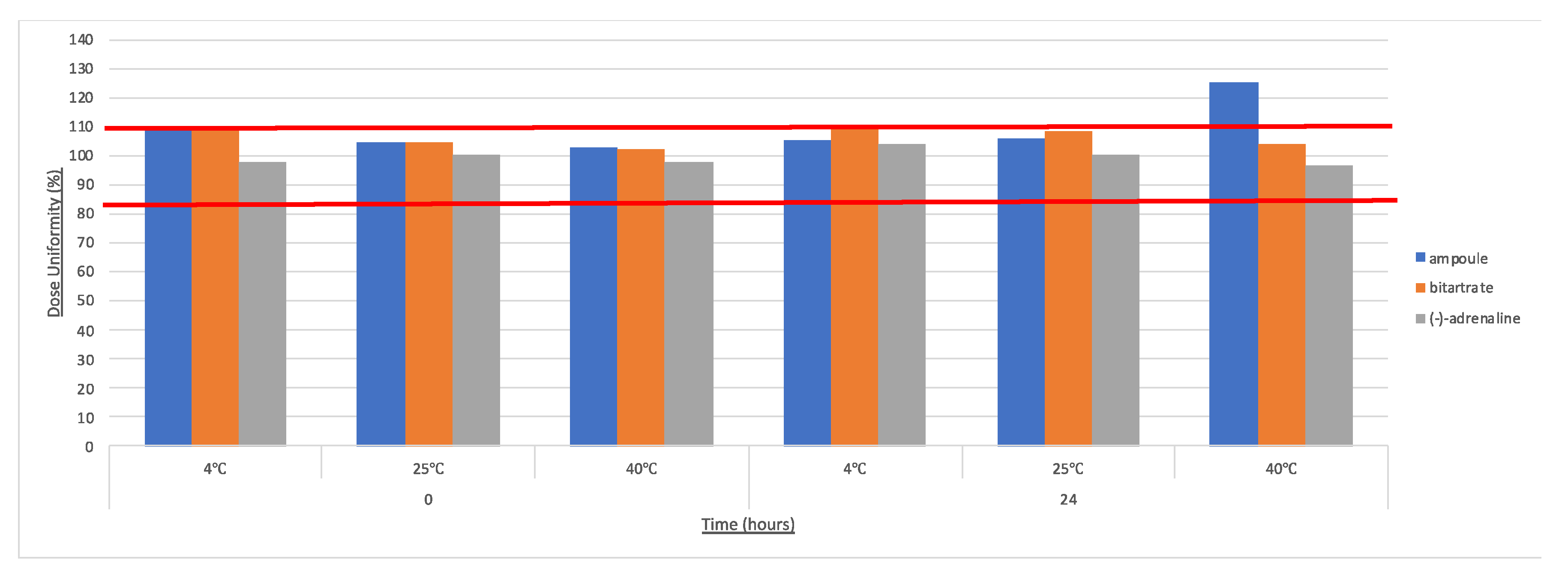
| Concentration and Dose Uniformity | Concentrations were calculated using absorbance readings and the Beer Lambert Law, ε280 = 2700 M−1 cm−1 and Mw = 183.2 g/mol. These were compared to the BP criteria, which states that adrenaline injection must contain 0.090 to 0.110% w/v and not less than 0.085% w/v is (−)-adrenaline. |
| pH Test | pH must be maintained between 2.8 and 4.0. |
| Formulation | ||||||
|---|---|---|---|---|---|---|
| (−)-Adrenaline | Bitartrate | Ampoule | ||||
| CD | UV | CD | UV | CD | UV | |
| Vibration (rpm) | ||||||
| 300 | 0.50 | 0.93 | - | - | - | - |
| 500 | 0.50 | 0.94 | - | - | - | - |
| 800 | 0.92 | 0.91 | 0.92 | 0.92 | 0.78 | 0.99 |
| Forced Degradation in Water | 5.47 × 10−16 | 1.26 × 10−8 | ||||
| Formulation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (−)-Adrenaline | Bitartrate | Ampoule | ||||||||
| CD | UV | CD | UV | CD | UV | |||||
| Time (hours) | 0.5 | 3 | 24 | 0.5 | 3 | 24 | 24 | 24 | 24 | 24 |
| Temperature (°C) | ||||||||||
| 4 | 0.45 | 0.06 | 0.22 | 0.72 | 0.99 | 0.99 | 0.64 | 0.92 | 0.89 | 0.88 |
| 25 | 0.70 | 0.98 | 0.81 | 0.98 | 0.94 | 0.98 | 0.59 | 0.91 | 0.79 | 0.93 |
| 40 | 0.02 | 0.94 | 0.23 | 0.98 | 0.96 | 0.99 | 0.90 | 0.90 | 0.35 | 0.16 |
| 65 | 0.36 | 0.91 | 0.97 | 0.87 | 0.45 | 0.77 | - | - | - | - |
| Forced Degradation in Water | 5.47 × 10−16 | 1.26 × 10−8 | ||||||||
| EpiPen® | ||||||
|---|---|---|---|---|---|---|
| EpiPen® A | EpiPen® B | EpiPen® C | ||||
| CD | UV | CD | UV | CD | UV | |
| p value | 0.55 | 0.97 | 0.11 | 0.98 | 0.73 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, S.; Bui, T.T.; Davies, A.; Courtney, P.; Brown, A.; Geudens, J.; Royall, P.G. An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns. Drones 2020, 4, 66. https://doi.org/10.3390/drones4040066
Beck S, Bui TT, Davies A, Courtney P, Brown A, Geudens J, Royall PG. An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns. Drones. 2020; 4(4):66. https://doi.org/10.3390/drones4040066
Chicago/Turabian StyleBeck, September, Tam T. Bui, Andrew Davies, Patrick Courtney, Alex Brown, Jef Geudens, and Paul G. Royall. 2020. "An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns" Drones 4, no. 4: 66. https://doi.org/10.3390/drones4040066
APA StyleBeck, S., Bui, T. T., Davies, A., Courtney, P., Brown, A., Geudens, J., & Royall, P. G. (2020). An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns. Drones, 4(4), 66. https://doi.org/10.3390/drones4040066









