Drone-Based High-Resolution Tracking of Aquatic Vertebrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Drone Piloting and Tracking
2.2. Measurement Accuracy
2.3. Data Preparation
2.4. Data Analysis
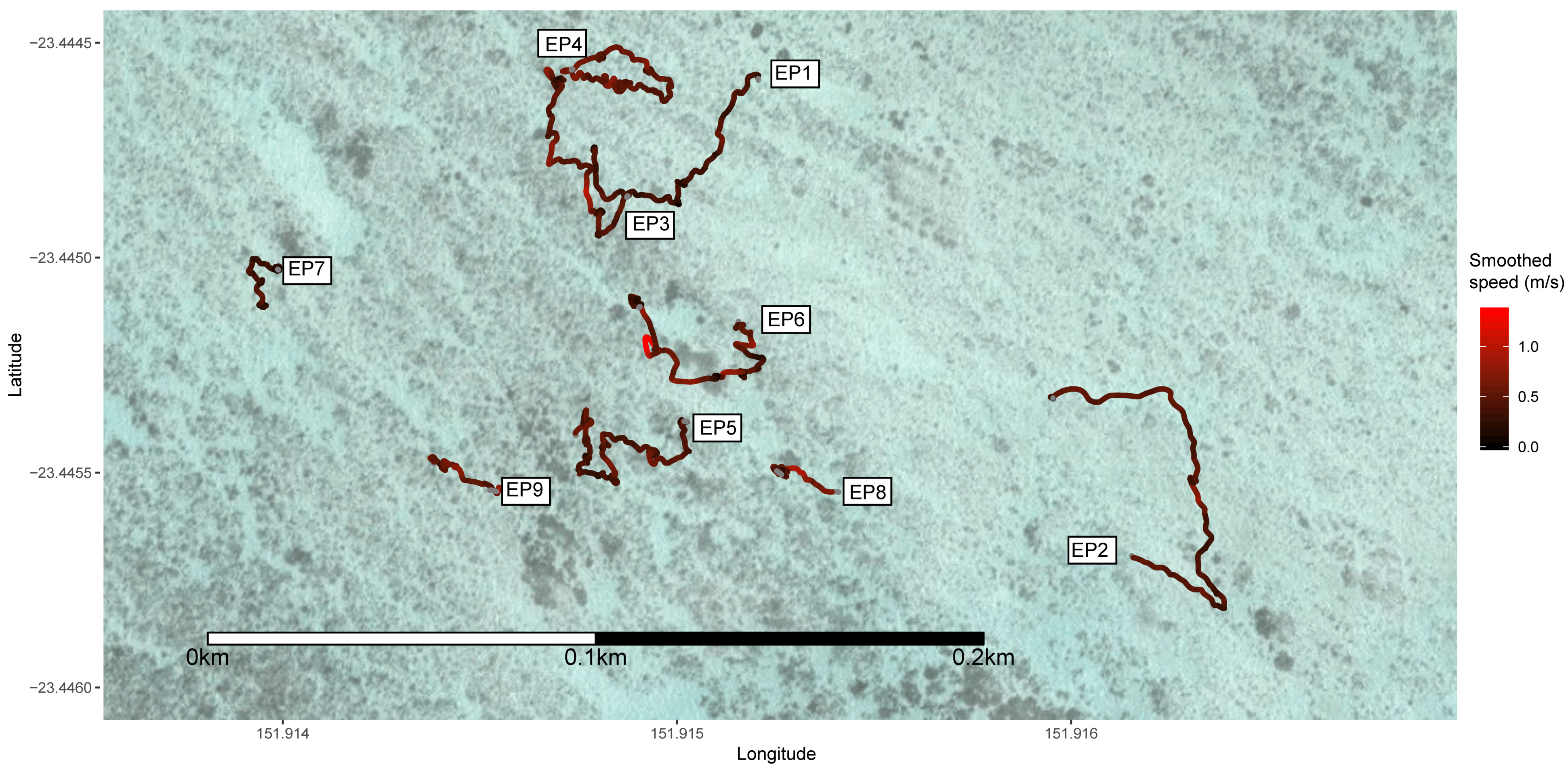
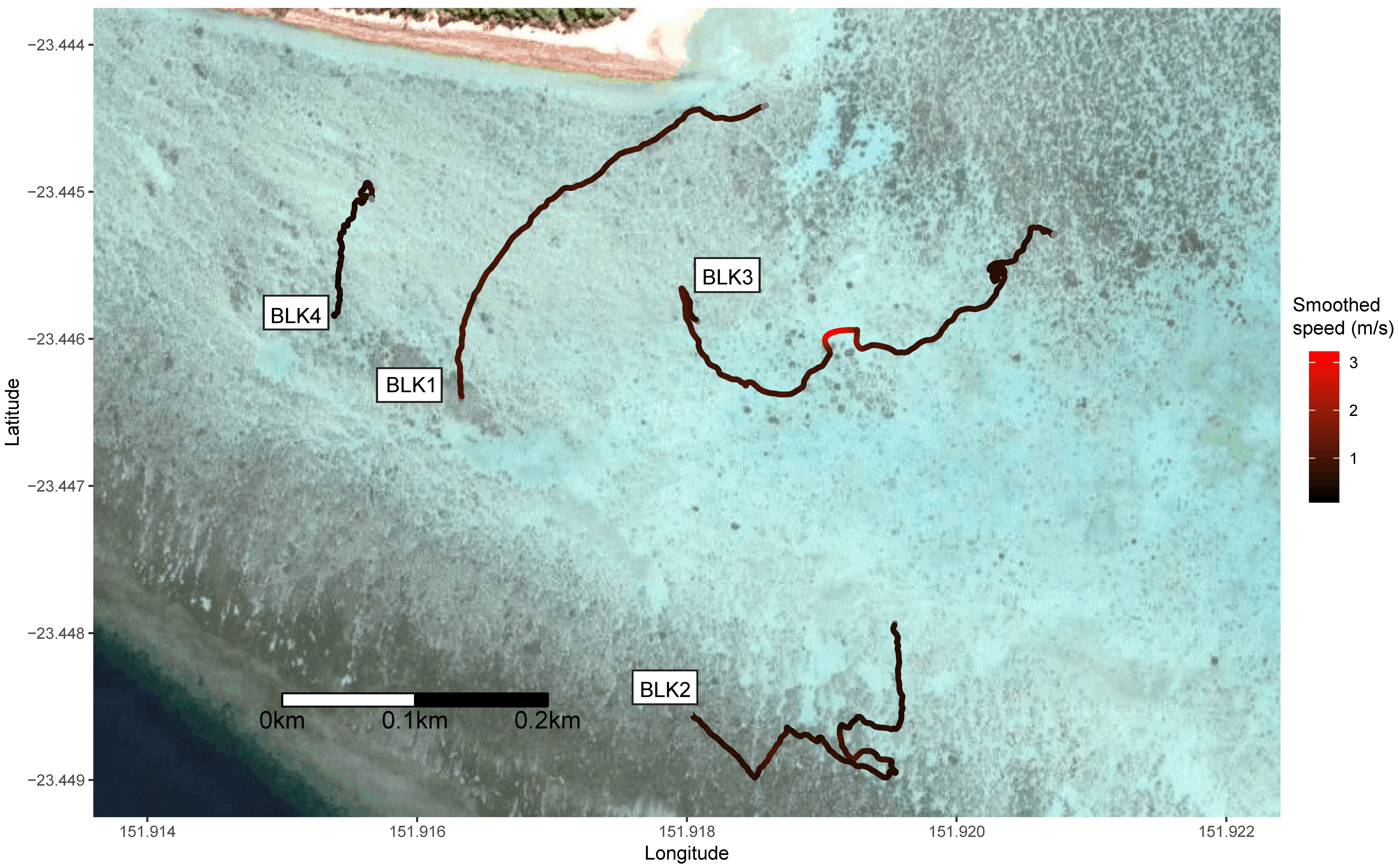
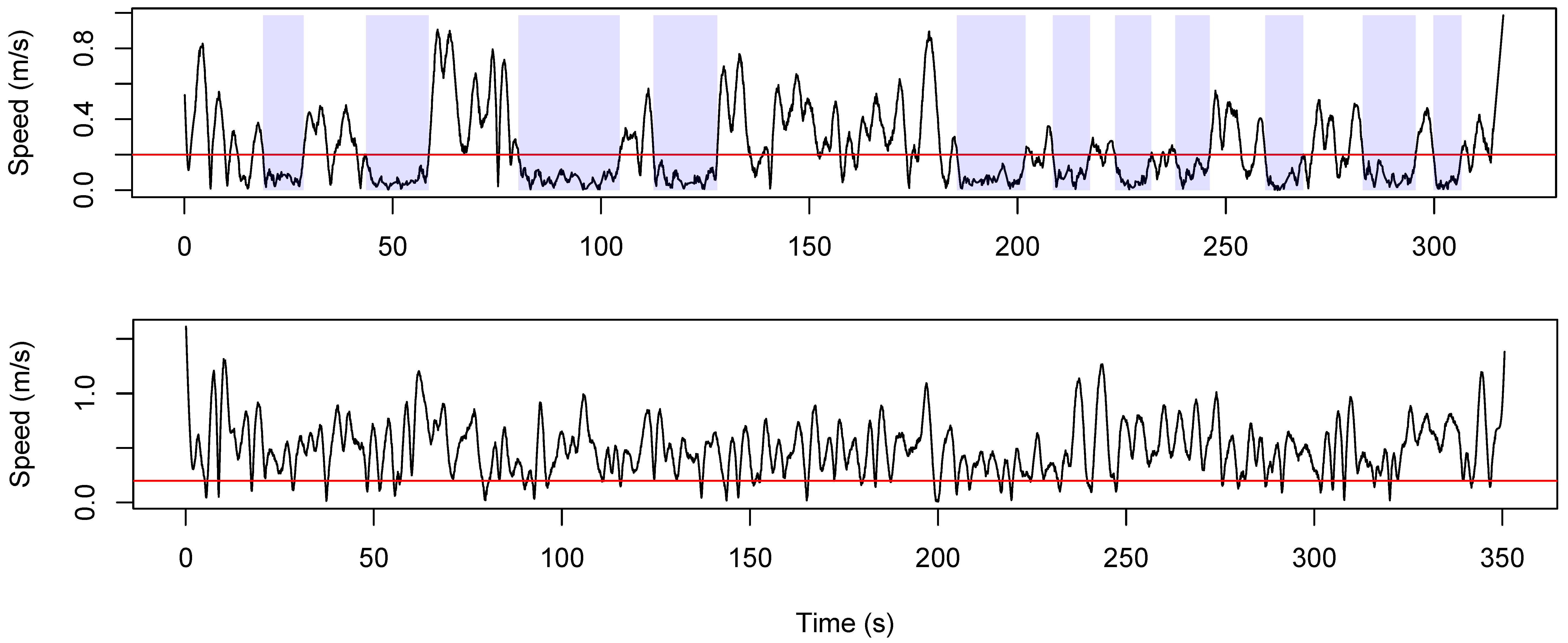
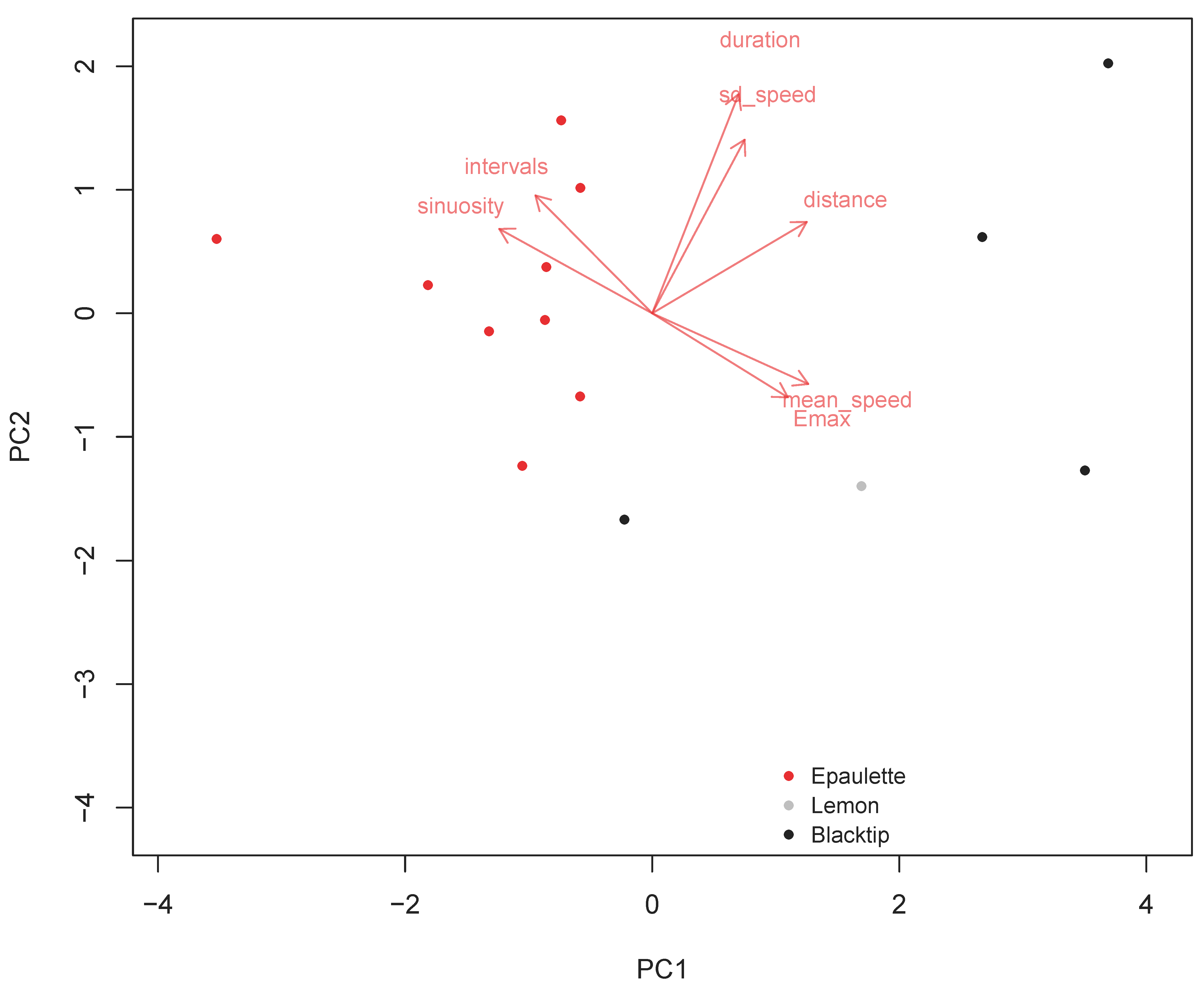
3. Results
4. Discussion
Considerations for Use of This Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nathan, R. An emerging movement ecology paradigm. Proc. Natl. Acad. Sci. USA 2008. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, T.G.; Gill, J.A.; Atkinson, P.W.; Gelinaud, G.; Potts, P.M.; Croger, R.E.; Gudmundsson, G.A.; Appleton, G.F.; Sutherland, W.J. Population-scale drivers of individual arrival times in migratory birds. J. Anim. Ecol. 2006, 75, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Fauchald, P.; Tveraa, T. Hierarchical patch dynamics and animal movement pattern. Oecologia 2006, 149, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Schofield, G.; Papafitsoros, K.; Haughey, R.; Katselidis, K. Aerial and underwater surveys reveal temporal variation in cleaning-station use by sea turtles at a temperate breeding area. Mar. Ecol. Prog. Ser. 2017, 575, 153–164. [Google Scholar] [CrossRef]
- Benhamou, S. How to reliably estimate the tortuosity of an animal’s path: Straightness, sinuosity, or fractal dimension? J. Theor. Biol. 2004, 229, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Clark, W.R.; Nusser, S.M.; Sovada, M.A.; Greenwood, R.J. Analysis of predator movement in prairie landscapes with contrasting grassland composition. J. Mammal. 2004, 85, 187–195. [Google Scholar] [CrossRef]
- Gilby, B.L.; Olds, A.D.; Connolly, R.M.; Stevens, T.; Henderson, C.J.; Maxwell, P.S.; Tibbetts, I.R.; Schoeman, D.S.; Rissik, D.; Schlacher, T.A. Optimising land-sea management for inshore coral reefs. PLoS ONE 2016, 11, e0164934. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.D.; Feldheim, K.A.; Papastamatiou, Y.P.; Hueter, R.E. There and back again: A review of residency and return migrations in sharks, with implications for population structure and management. Annu. Rev. Mar. Sci. 2015, 7, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Braccini, M.; Aires-da-Silva, A.; Taylor, I. Incorporating movement in the modelling of shark and ray population dynamics: Approaches and management implications. Rev. Fish. Biol. Fish. 2016, 26, 13–24. [Google Scholar] [CrossRef]
- Taylor, M.D.; Babcock, R.C.; Simpfendorfer, C.A.; Crook, D.A. Where technology meets ecology: Acoustic telemetry in contemporary Australian aquatic research and management. Mar. Freshw. Res. 2017, 68, 1397–1402. [Google Scholar] [CrossRef]
- Hart, C.E.; Blanco, G.S.; Coyne, M.S.; Delgado-Trejo, C.; Godley, B.J.; Jones, T.T.; Resendiz, A.; Seminoff, J.A.; Witt, M.J.; Nichols, W.J. Multinational tagging efforts illustrate regional scale of distribution and threats for east pacific green turtles (Chelonia mydas agassizii). PLoS ONE 2015, 10, e0116225. [Google Scholar] [CrossRef] [PubMed]
- Bestley, S.; Jonsen, I.D.; Hindell, M.A.; Harcourt, R.G.; Gales, N.J. Taking animal tracking to new depths: Synthesizing horizontal–vertical movement relationships for four marine predators. Ecology 2015, 96, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.C.; Foley, D.G.; Ganong, J.E.; Perle, C.; Shillinger, G.L.; Block, B.A. Migration of an upper trophic level predator, the salmon shark Lamna ditropis, between distant ecoregions. Mar. Ecol. Prog. Ser. 2008, 372, 253–264. [Google Scholar] [CrossRef]
- Gore, M.A.; Rowat, D.; Hall, J.; Gell, F.R.; Ormond, R.F. Transatlantic migration and deep mid-ocean diving by basking shark. Biol. Lett. 2008, 4, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, J.J.; Byrne, M.E.; Wetherbee, B.M.; Harvey, G.M.; Shivji, M.S. Long-term satellite tracking reveals region-specific movements of a large pelagic predator, the shortfin mako shark, in the western north Atlantic Ocean. J. Appl. Ecol. 2017, 54, 1765–1775. [Google Scholar] [CrossRef]
- Domeier, M.L.; Nasby-Lucas, N. Two-year migration of adult female white sharks (Carcharodon carcharias) reveals widely separated nursery areas and conservation concerns. Anim. Biotelem. 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Heupel, M.; Simpfendorfer, C. Using acoustic monitoring to evaluate MPAS for shark nursery areas: The importance of long-term data. Mar. Technol. Soc. J. 2005, 39, 10–18. [Google Scholar] [CrossRef]
- Garrigue, C.; Clapham, P.J.; Geyer, Y.; Kennedy, A.S.; Zerbini, A.N. Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered south pacific humpback whales. R. Soc. Open Sci. 2015, 2, 150489. [Google Scholar] [CrossRef] [PubMed]
- Heupel, M.R.; Lédée, E.J.; Simpfendorfer, C.A. Telemetry reveals spatial separation of co-occurring reef sharks. Mar. Ecol. Prog. Ser. 2018, 589, 179–192. [Google Scholar] [CrossRef]
- Mourier, J.; Maynard, J.; Parravicini, V.; Ballesta, L.; Clua, E.; Domeier, M.L.; Planes, S. Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Curr. Biol. 2016, 26, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Brough, T.; Rayment, W.; Slooten, E.; Dawson, S. Fine scale distribution for a population of New Zealand’s only endemic dolphin (Cephalorhynchus hectori) shows long-term stability of coastal hotspots. Mar. Mamm. Sci. 2018. [Google Scholar] [CrossRef]
- Meyer, C.G.; Anderson, J.M.; Coffey, D.M.; Hutchinson, M.R.; Royer, M.A.; Holland, K.N. Habitat geography around Hawaii’s oceanic islands influences tiger shark (Galeocerdo cuvier) spatial behaviour and shark bite risk at ocean recreation sites. Sci. Rep. 2018, 8, 4945. [Google Scholar] [CrossRef] [PubMed]
- Kock, A.A.; Photopoulou, T.; Durbach, I.; Mauff, K.; Meÿer, M.; Kotze, D.; Griffiths, C.L.; O’Riain, M.J. Summer at the beach: Spatio-temporal patterns of white shark occurrence along the inshore areas of false bay, South Africa. Mov. Ecol. 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Tyminski, J.P.; de la Parra-Venegas, R.; Cano, J.G.; Hueter, R.E. Vertical movements and patterns in diving behavior of whale sharks as revealed by pop-up satellite tags in the eastern gulf of Mexico. PLoS ONE 2015, 10, e0142156. [Google Scholar] [CrossRef] [PubMed]
- Schlaff, A.M.; Heupel, M.R.; Udyawer, V.; Simpfendorfer, C.A. Biological and environmental effects on activity space of a common reef shark on an inshore reef. Mar. Ecol. Prog. Ser. 2017, 571, 169–181. [Google Scholar] [CrossRef]
- Armansin, N.; Lee, K.; Huveneers, C.; Harcourt, R. Integrating social network analysis and fine-scale positioning to characterize the associations of a benthic shark. Anim. Behav. 2016, 115, 245–258. [Google Scholar] [CrossRef]
- Johnson, M.; de Soto, N.A.; Madsen, P.T. Studying the behaviour and sensory ecology of marine mammals using acoustic recording tags: A review. Mar. Ecol. Prog. Ser. 2009, 395, 55–73. [Google Scholar] [CrossRef]
- Wilson, A.D.; Brownscombe, J.W.; Krause, J.; Krause, S.; Gutowsky, L.F.; Brooks, E.J.; Cooke, S.J. Integrating network analysis, sensor tags, and observation to understand shark ecology and behavior. Behav. Ecol. 2015, 26, 1577–1586. [Google Scholar] [CrossRef]
- Wilson, R.P.; Liebsch, N.; Davies, I.M.; Quintana, F.; Weimerskirch, H.; Storch, S.; Lucke, K.; Siebert, U.; Zankl, S.; Müller, G. All at sea with animal tracks; methodological and analytical solutions for the resolution of movement. Deep Sea Res. Part II 2007, 54, 193–210. [Google Scholar] [CrossRef]
- Huveneers, C.; Simpfendorfer, C.A.; Kim, S.; Semmens, J.M.; Hobday, A.J.; Pederson, H.; Stieglitz, T.; Vallee, R.; Webber, D.; Heupel, M.R. The influence of environmental parameters on the performance and detection range of acoustic receivers. Methods Ecol. Evol. 2016, 7, 825–835. [Google Scholar] [CrossRef]
- Gunn, J.; Stevens, J.; Davis, T.; Norman, B. Observations on the short-term movements and behaviour of whale sharks (Rhincodon typus) at ningaloo reef, Western Australia. Mar. Biol. 1999, 135, 553–559. [Google Scholar] [CrossRef]
- Carlisle, A.B.; Starr, R.M. Habitat use, residency, and seasonal distribution of female leopard sharks Triakis semifasciata in Elkhorn Slough, California. Mar. Ecol. Prog. Ser. 2009, 380, 213–228. [Google Scholar] [CrossRef]
- Scales, K.L.; Lewis, J.; Lewis, J.; Castellanos, D.; Godley, B.; Graham, R. Insights into habitat utilisation of the hawksbill turtle, Eretmochelys imbricata (linnaeus, 1766), using acoustic telemetry. J. Exp. Mar. Biol. Ecol. 2011, 407, 122–129. [Google Scholar] [CrossRef]
- Bouyoucos, I.; Suski, C.; Mandelman, J.; Brooks, E. Effect of weight and frontal area of external telemetry packages on the kinematics, activity levels and swimming performance of small-bodied sharks. J. Fish. Biol. 2017, 90, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Guttridge, T.; Cowx, I.; Elliott, M.; Gruber, S. The behaviour and recovery of juvenile lemon sharks Negaprion brevirostris in response to external accelerometer tag attachment. J. Fish. Biol. 2015, 87, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Hays, G.C.; Ferreira, L.C.; Sequeira, A.M.; Meekan, M.G.; Duarte, C.M.; Bailey, H.; Bailleul, F.; Bowen, W.D.; Caley, M.J.; Costa, D.P. Key questions in marine megafauna movement ecology. Trends Ecol. Evol. 2016, 31, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Gendron, D.; Serrano, I.M.; de la Cruz, A.U.; Calambokidis, J.; Mate, B. Long-term individual sighting history database: An effective tool to monitor satellite tag effects on cetaceans. Endanger. Spec. Res. 2015, 26, 235–241. [Google Scholar] [CrossRef]
- Fontes, J.; Baeyaert, J.; Prieto, R.; Graça, G.; Buyle, F.; Afonso, P. New non-invasive methods for short-term electronic tagging of pelagic sharks and rays. Mar. Biol. 2018, 165, 34. [Google Scholar] [CrossRef]
- Fregosi, S.; Klinck, H.; Horning, M.; Costa, D.P.; Mann, D.; Sexton, K.; Hückstädt, L.A.; Mellinger, D.K.; Southall, B.L. An animal-borne active acoustic tag for minimally invasive behavioral response studies on marine mammals. Anim. Biotelem. 2016, 4, 9. [Google Scholar] [CrossRef]
- Pirotta, V.; Smith, A.; Ostrowski, M.; Russell, D.; Jonsen, I.D.; Grech, A.; Harcourt, R. An economical custom-built drone for assessing whale health. Front. Mar. Sci. 2017, 4, 425. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Papastamatiou, Y.P.; Barnett, A. Apex predatory sharks and crocodiles simultaneously scavenge a whale carcass. J. Ethol. 2018, 36, 205–209. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P.; Browman, H.E.H. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2017, 75, 1–8. [Google Scholar] [CrossRef]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Rieucau, G.; Kiszka, J.J.; Castillo, J.C.; Mourier, J.; Boswell, K.M.; Heithaus, M.R. Using unmanned aerial vehicle (UAV) surveys and image analysis in the study of large surface-associated marine species: A case study on reef sharks Carcharhinus melanopterus shoaling behaviour. J. Fish. Biol. 2018, 93, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Abels, L.; Wasik, S.; Saddler, L.; Ormond, R. Are close-following and breaching behaviours by basking sharks at aggregation sites related to courtship? J. Mar. Biol. Assoc. UK 2018, 1–13. [Google Scholar] [CrossRef]
- Cárdenas, N.Y.; Joyce, K.E.; Maier, S.W. Monitoring mangrove forests: Are we taking full advantage of technology? Int. J. Appl. Earth Obs. Geoinf. 2017, 63, 1–14. [Google Scholar] [CrossRef]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO Publishing: Collingwood, Australia, 2009. [Google Scholar]
- Routley, M.H.; Nilsson, G.E.; Renshaw, G.M. Exposure to hypoxia primes the respiratory and metabolic responses of the epaulette shark to progressive hypoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 313–321. [Google Scholar] [CrossRef]
- Wise, G.; Mulvey, J.M.; Renshaw, G.M. Hypoxia tolerance in the epaulette shark (Hemiscyllium ocellatum). J. Exp. Zool. 1998, 281, 1–5. [Google Scholar] [CrossRef]
- Hickey, A.J.; Renshaw, G.M.; Speers-Roesch, B.; Richards, J.G.; Wang, Y.; Farrell, A.P.; Brauner, C.J. A radical approach to beating hypoxia: Depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). J. Comp. Physiol. B 2012, 182, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Heupel, M.; Bennett, M. Observations on the diet and feeding habits of the epaulette shark, Hemiscyllium ocellatum (bonnaterre), on heron island reef, Great Barrier Reef, Australia. Mar. Freshw. Res. 1998, 49, 753–756. [Google Scholar] [CrossRef]
- Heupel, M.; Bennett, M. Estimating abundance of reef-dwelling sharks: A case study of the epaulette shark, Hemiscyllium ocellatum (elasmobranchii: Hemiscyllidae). Pac. Sci. 2007, 61, 383–394. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Lowe, C.G.; Caselle, J.E.; Friedlander, A.M. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 2009, 90, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.S.; Veríssimo, A.; Magozzi, S.; Abrantes, K.G.; Aguilar, A.; Al-Reasi, H.; Barnett, A.; Bethea, D.M.; Biais, G.; Borrell, A. A global perspective on the trophic geography of sharks. Nat. Ecol. Evol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.J.; Shiffman, D.S.; Byrnes, E.E.; Hammerschlag-Peyer, C.; Hammerschlag, N. Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat. Ecol. 2017, 51, 435–448. [Google Scholar] [CrossRef]
- Guttridge, T.L.; van Dijk, S.; Stamhuis, E.J.; Krause, J.; Gruber, S.H.; Brown, C. Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim. Cogn. 2013, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.E.; Byrnes, E.E.; Clark, J.A.; Connolly, D.M.; Schiller, S.E.; Thompson, J.A.; Tosetto, L.; Martinelli, J.C.; Raoult, V. Ecological impacts and management implications of reef walking on a tropical reef flat community. Mar. Pollut. Bull. 2017, 114, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Gennari, E.; Cowley, P.D.; Johnson, R.L. Performance and reliability of active acoustic biotelemetry to best track marine pelagic species in temperate coastal waters. Mar. Biol. 2018, 165, 128. [Google Scholar] [CrossRef]
- Hugenholtz, C.; Brown, O.; Walker, J.; Barchyn, T.; Nesbit, P.; Kucharczyk, M.; Myshak, S. Spatial accuracy of UAV-derived orthoimagery and topography: Comparing photogrammetric models processed with direct geo-referencing and ground control points. Geomatica 2016, 70, 21–30. [Google Scholar] [CrossRef]
- Ferraript. Txtlogtocsvtool. 2018. Available online: https://phantompilots.com/attachments/txtlogtocsvtool-zip.98013/ (accessed on 21 April 2018).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Khale, D.; Wickham, H. Ggmap: Spatial visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar]
- Michelot, T.; Langrock, R.; Patterson, T.A. Movehmm: An r package for the statistical modelling of animal movement data using hidden markov models. Methods Ecol. Evol. 2016, 7, 1308–1315. [Google Scholar] [CrossRef]
- Albertsen, C.M.; Whoriskey, K.; Yurkowski, D.; Nielsen, A.; Flemming, J.M. Fast fitting of non-gaussian state-space models to animal movement data via template model builder. Ecology 2015, 96, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.J.; Skowron Volponi, M.A. Trajr: An r package for characterisation of animal trajectories. Ethology 2018. [Google Scholar] [CrossRef]
- Espinoza, M.; Farrugia, T.J.; Webber, D.M.; Smith, F.; Lowe, C.G. Testing a new acoustic telemetry technique to quantify long-term, fine-scale movements of aquatic animals. Fish. Res. 2011, 108, 364–371. [Google Scholar] [CrossRef]
- Guzzo, M.M.; Van Leeuwen, T.E.; Hollins, J.; Koeck, B.; Newton, M.; Webber, D.M.; Smith, F.I.; Bailey, D.M.; Killen, S.S. Field testing a novel high residence positioning system for monitoring the fine-scale movements of aquatic organisms. Methods Ecol. Evol. 2018, 9, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Wich, S.A.; Koh, L.P. Conservation Drones: Mapping and Monitoring Biodiversity; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Heithaus, M.R.; Marshall, G.J.; Buhleier, B.M.; Dill, L.M. Employing crittercam to study habitat use and behavior of large sharks. Mar. Ecol. Prog. Ser. 2001, 209, 307–310. [Google Scholar] [CrossRef]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Front. Mar. Sci. 2016, 3, 277. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Duce, S.; Leahy, S.; Leon, J.X.; Maier, S. Principles and practice of acquiring drone based image data in marine environments. Mar. Freshw. Res. 2018. [Google Scholar] [CrossRef]

| Step | Process |
|---|---|
| 1 | Check for appropriate weather conditions (low wind <10 kph = optimal, maximum of 30 kph). |
| 2 | Take off with drone and fully-charged battery, fly with camera pointed directly down. |
| 3 | Fly S-shaped search pattern above area of interest at 15–50 m altitude, depending on size of target species. Adjust height accordingly. |
| 4 | Once target species is spotted, immediately centre animal in the dead centre of viewing screen and descend to 2–5 m altitude. |
| 5 | Start recording video to record behaviours and aid in data processing, |
| 6 | Use gentle and consistent pilot inputs to remain directly above animal in this fashion. Avoid jagged and large inputs (this is true of any remote piloting, but especially in this case). |
| 7 | Continue tracking until animal is lost or battery gets to the 30% mark (this will prevent long-term battery degradation). |
| 8 | Return drone and land. |
| Track Number | Species | Estimated Length (m) | Duration of Track (s) | Distance Travelled (m) | Mean Speed (m/s) | SD of Speed (m/s) | Sinuosity | Emax (103) | Pauses Greater than 5 s (/min) | Observed Foraging |
|---|---|---|---|---|---|---|---|---|---|---|
| EP1 | Epaulette | <0.7 | 406.3 | 116 | 0.29 | 0.21 | 0.92 | 4.36 | 1.03 | Yes |
| EP2 | Epaulette | <0.7 | 377.7 | 160 | 0.42 | 0.25 | 0.81 | 6.34 | 0.48 | No |
| EP3 | Epaulette | <0.7 | 429.5 | 172 | 0.40 | 0.26 | 0.87 | 5.99 | 0.56 | No |
| EP4 | Epaulette | <0.7 | 350.6 | 169 | 0.48 | 0.23 | 0.80 | 7.56 | 0 | No |
| EP5 | Epaulette | <0.7 | 602.8 | 222 | 0.37 | 0.23 | 0.95 | 4.85 | 0.40 | No |
| EP6 | Epaulette | <0.7 | 468.9 | 179 | 0.38 | 0.36 | 0.97 | 4.50 | 0.90 | No |
| EP7 | Epaulette | <0.7 | 316.6 | 69 | 0.22 | 0.19 | 1.46 | 2.12 | 2.08 | No |
| EP8 | Epaulette | <0.7 | 301.8 | 117 | 0.39 | 0.28 | 1.04 | 4.29 | 0.40 | No |
| EP9 | Epaulette | <0.7 | 185.7 | 89 | 0.48 | 0.27 | 0.86 | 5.99 | 0 | No |
| BLK1 | Blacktip | >1.25 | 378.7 | 386 | 1.02 | 0.31 | 0.14 | 226.91 | 0 | No |
| BLK2 | Blacktip | >1.25 | 680.6 | 454 | 0.67 | 0.26 | 0.19 | 116.03 | 0 | No |
| BLK3 | Blacktip | >1.25 | 645.5 | 562 | 0.87 | 0.45 | 0.26 | 69.24 | 0 | Yes |
| BLK4 | Blacktip | >1.25 | 300.2 | 161 | 0.54 | 0.17 | 0.40 | 29.13 | 0 | Yes |
| LEM1 | Lemon | >1.25 | 357.4 | 286 | 0.80 | 0.23 | 0.20 | 107.39 | 0 | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoult, V.; Tosetto, L.; Williamson, J.E. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones 2018, 2, 37. https://doi.org/10.3390/drones2040037
Raoult V, Tosetto L, Williamson JE. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones. 2018; 2(4):37. https://doi.org/10.3390/drones2040037
Chicago/Turabian StyleRaoult, Vincent, Louise Tosetto, and Jane E. Williamson. 2018. "Drone-Based High-Resolution Tracking of Aquatic Vertebrates" Drones 2, no. 4: 37. https://doi.org/10.3390/drones2040037
APA StyleRaoult, V., Tosetto, L., & Williamson, J. E. (2018). Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones, 2(4), 37. https://doi.org/10.3390/drones2040037






