Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images
Abstract
:1. Introduction
2. Materials and Methods
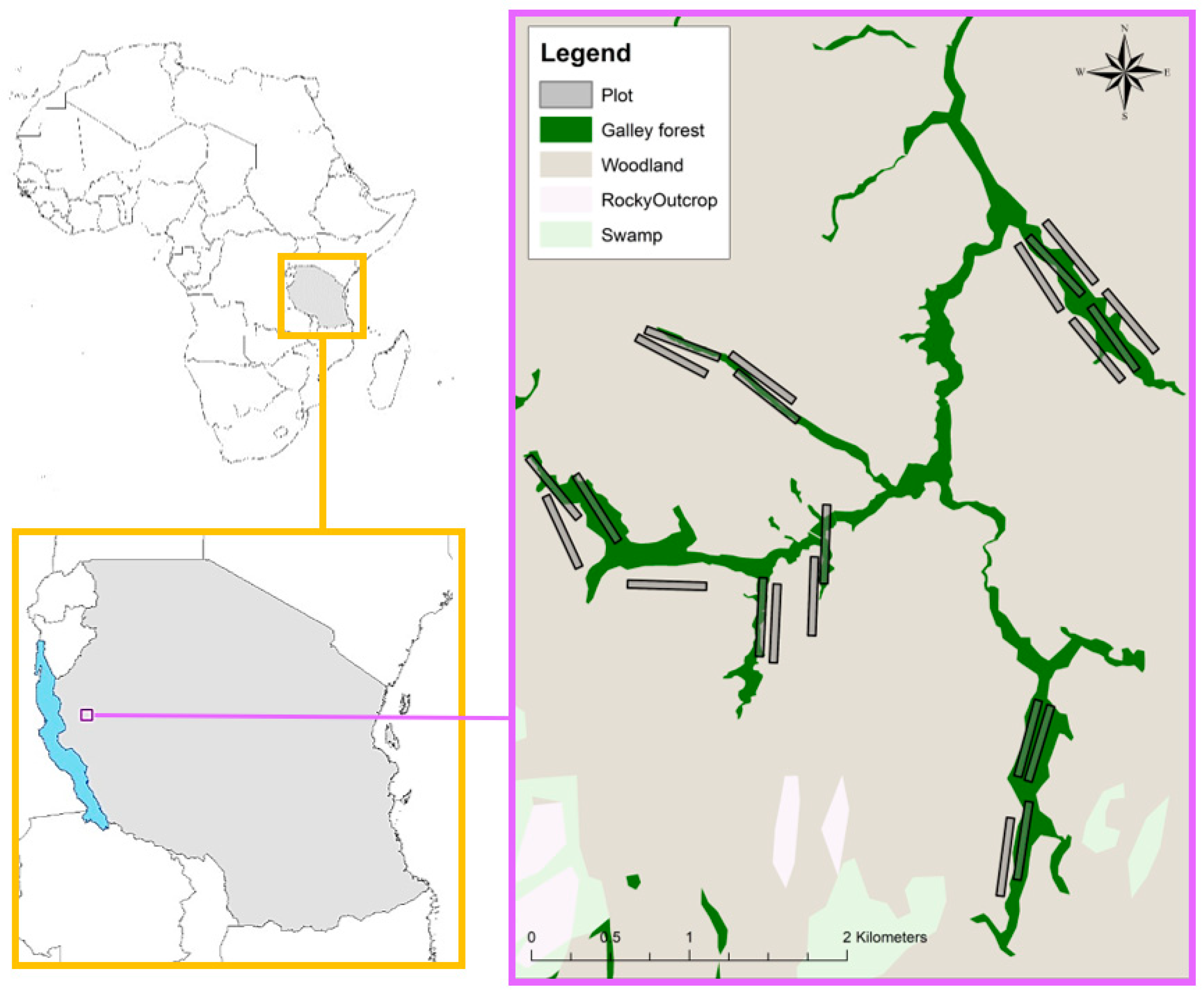
2.1. Study Site
2.2. Ground Surveys
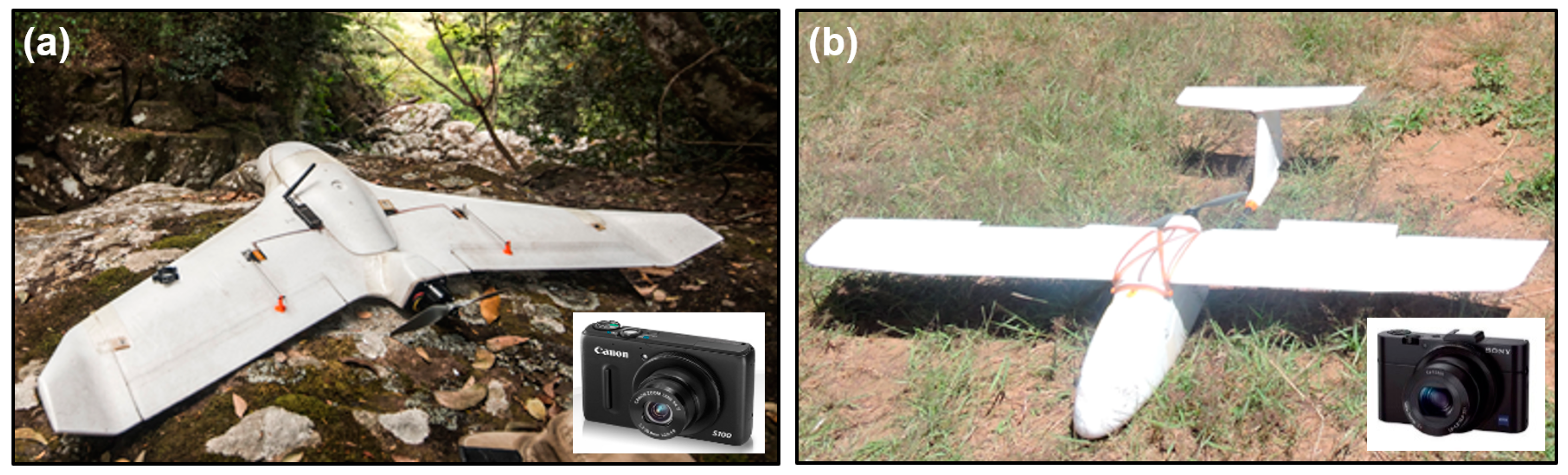
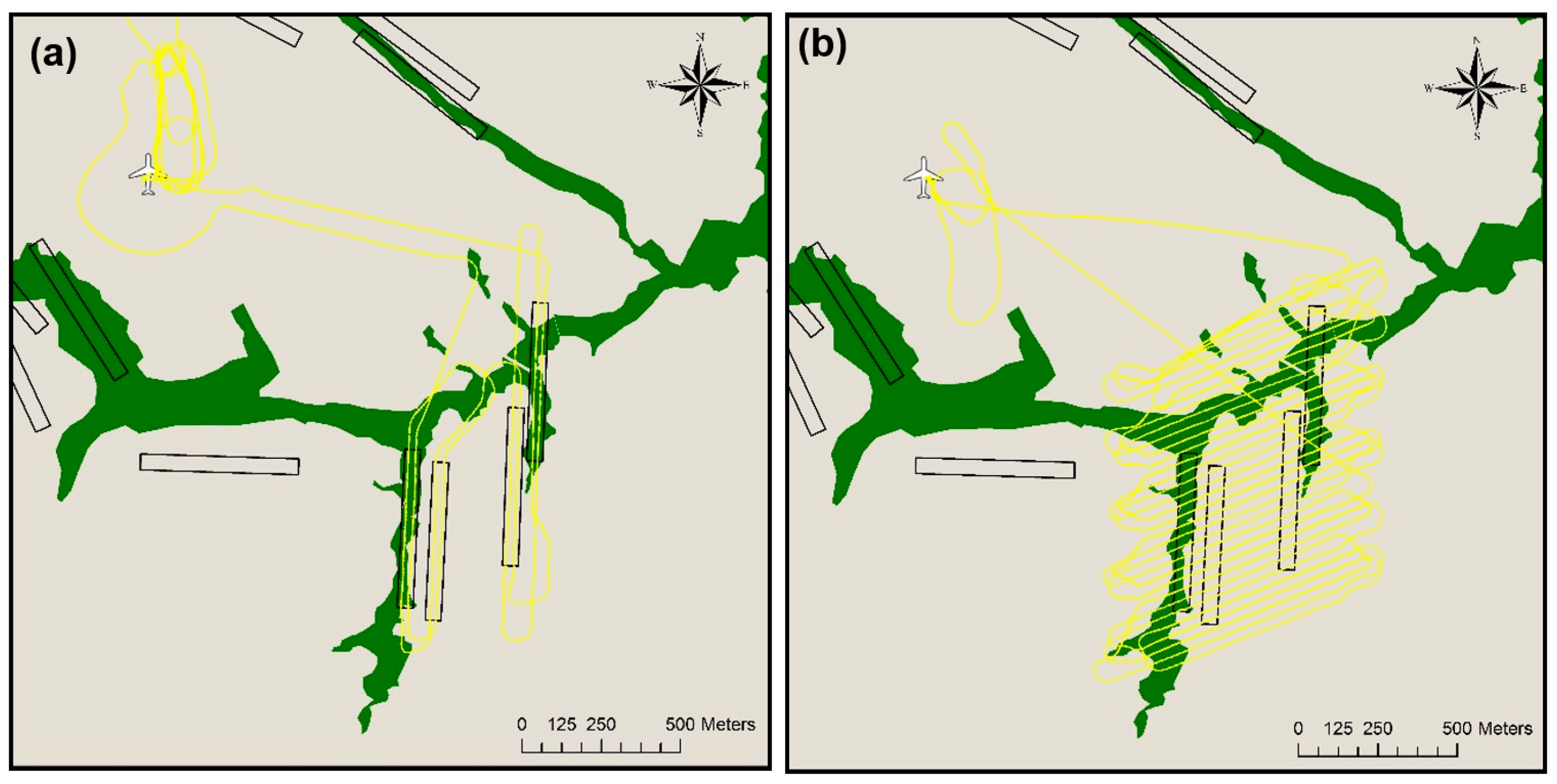
2.3. Aerial Surveys
2.4. Nest Detection
2.5. Analyses
2.5.1. Performance of the Aerial Detection
2.5.2. Factors Influencing Detectability
3. Results
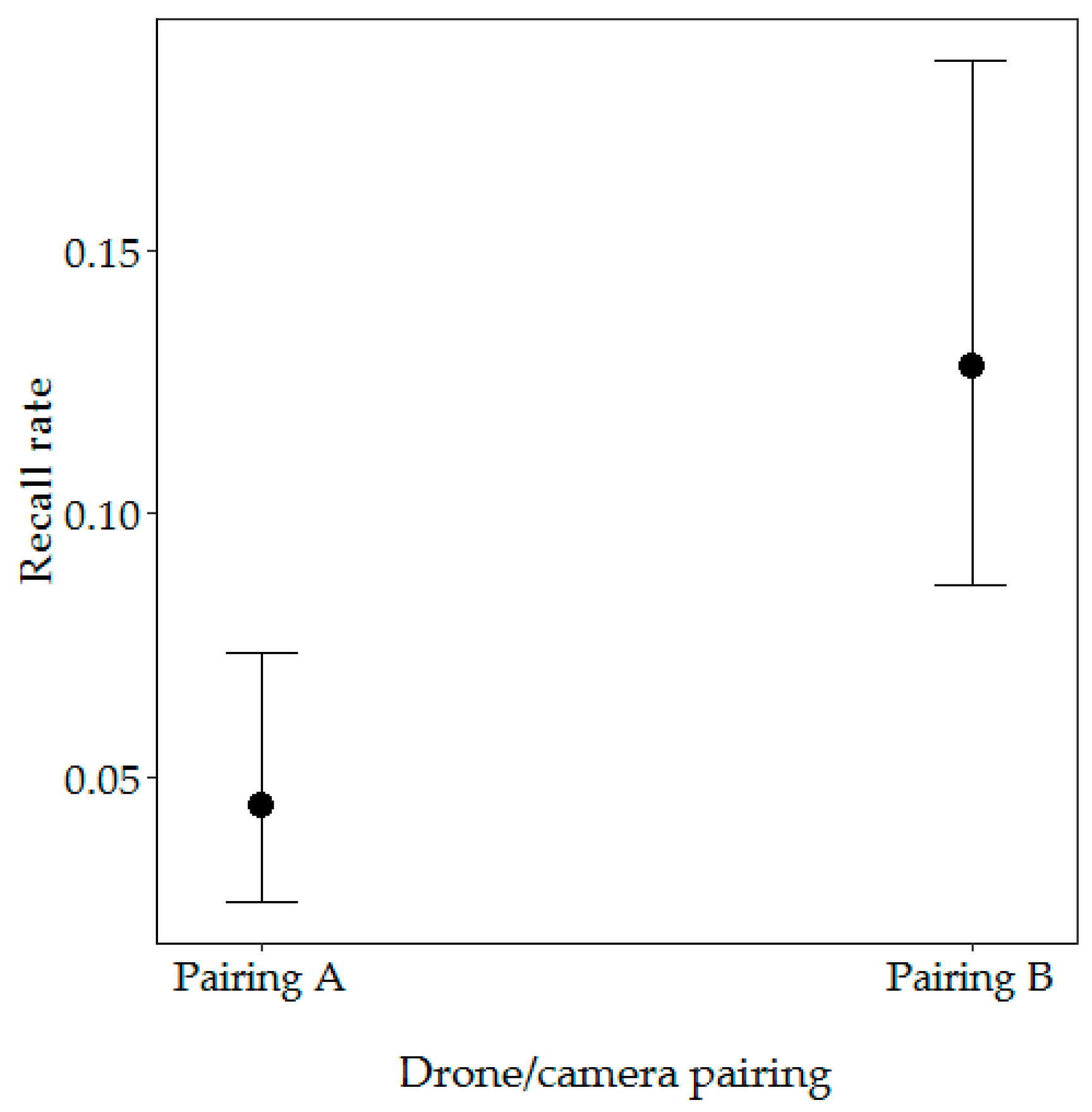
3.1. Performance of the Aerial Detection
3.2. Factors Influencing Detectability
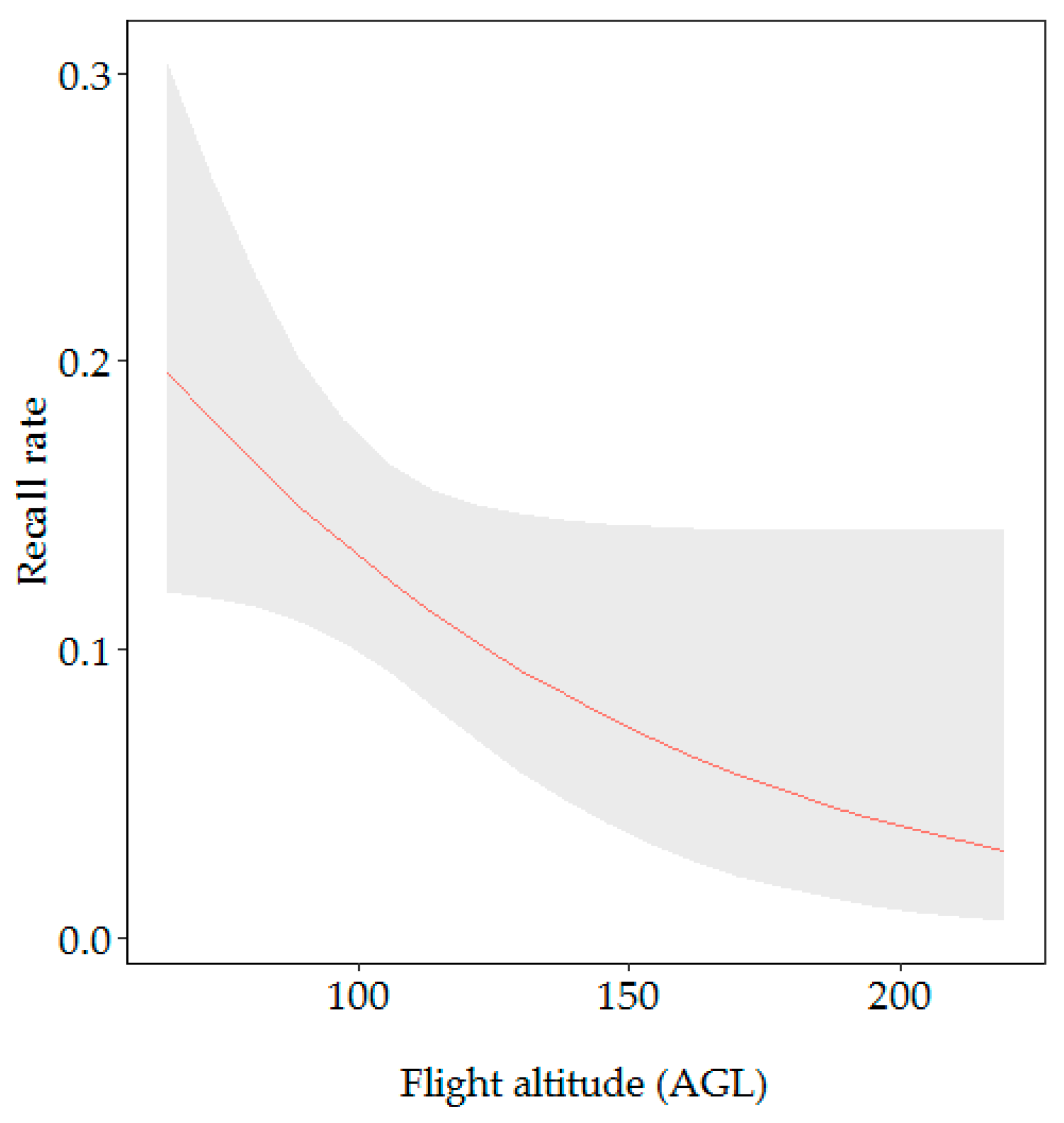
3.2.1. Factors Influencing the Recall Rate
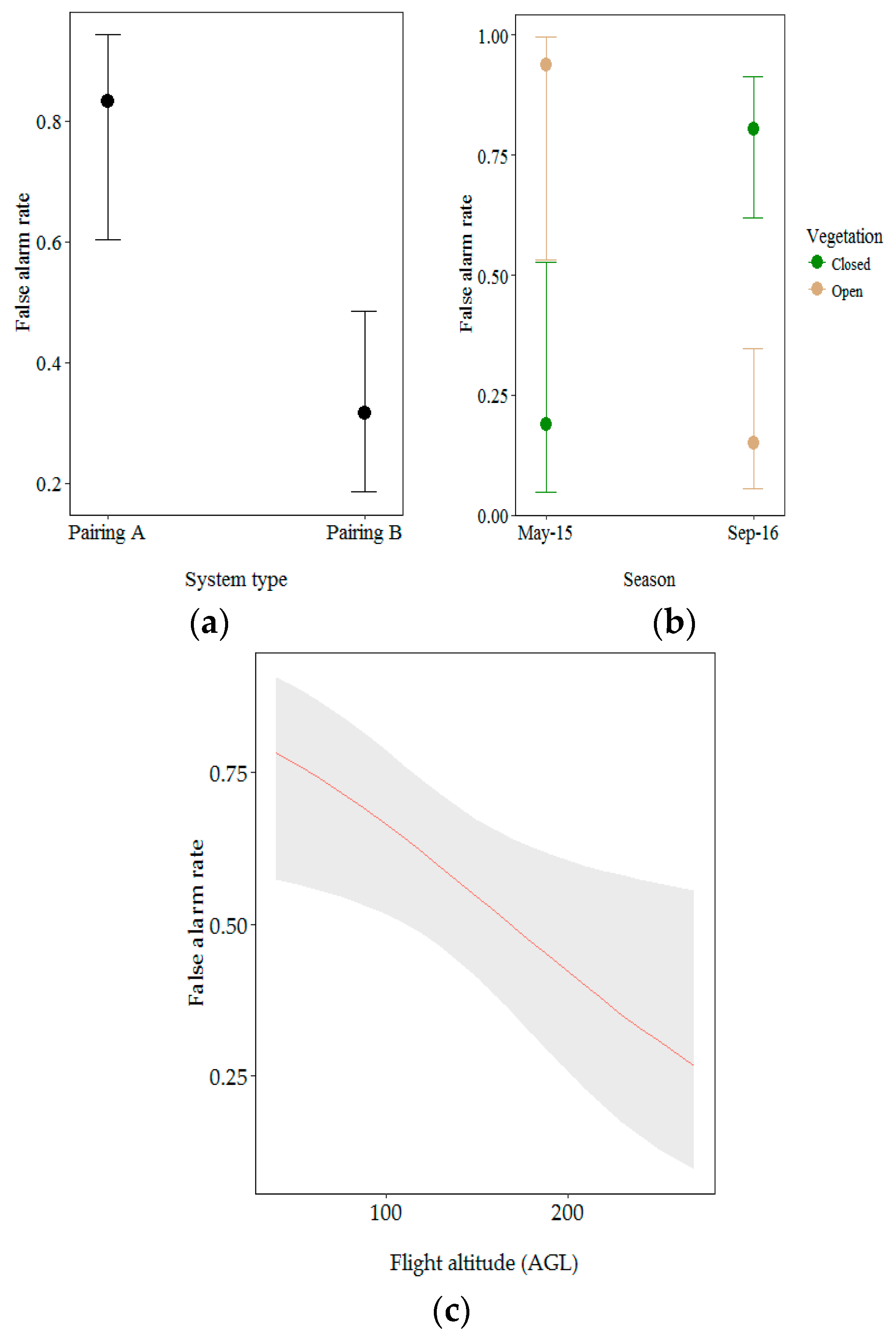
3.2.2. Factors Influencing the False Alarm Rate
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species. Version 2017-2. 2017. Available online: http://www.iucnredlist.org (accessed on 21 September 2017).
- Campbell, G.; Kuehl, H.; N’Goran Kouamé, P.; Boesch, C. Alarming decline of West African chimpanzees in Côte d’Ivoire. Curr. Biol. 2008, 18, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.; Blake, S.; Boesch, C.; Campbell, G.; du Toit, L.; Duvall, C.; Ekobo, A.; Etoga, G.; Galat-Luong, A.; Gamys, J.; et al. Recent decline in suitable environmental conditions for African great apes. Divers. Distrib. 2012, 18, 1077–1091. [Google Scholar] [CrossRef]
- Wich, S.A.; Garcia-Ulloa, J.; Kühl, H.S.; Humle, T.; Lee, J.S.H.; Koh, L.P. Will oil palm’s homecoming spell doom for Africa’s great apes? Curr. Biol. 2014, 24, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Jones, E.; Pendry, S. The threat to primates and other mammals from the bushmeat trade in Africa, and how this threat could be diminished. Oryx 1999, 33, 233–246. [Google Scholar] [CrossRef]
- McLennan, M.R.; Hyeroba, D.; Asiimwe, C.; Reynolds, V.; Wallis, J. Chimpanzees in mantraps: lethal crop protection and conservation in Uganda. Oryx 2012, 46, 598–603. [Google Scholar] [CrossRef]
- Piel, A.K.; Lenoel, A.; Johnson, C.; Stewart, F.A. Deterring poaching in western Tanzania: The presence of wildlife researchers. Glob. Ecol. Conserv. 2015, 3, 188–199. [Google Scholar] [CrossRef]
- Walsh, P.D.; Abernethy, K.A.; Bermejo, M.; Beyers, R.; De Wachter, P.; Akou, M.E.; Huijbregts, B.; Mambounga, D.I.; Toham, A.K.; Kilbourn, A.M.; et al. Catastrophic ape decline in western equatorial Africa. Nature 2003, 422, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Rudicell, R.S.; Holland Jones, J.; Wroblewski, E.E.; Learn, G.H.; Li, Y.; Robertson, J.D.; Greengrass, E.; Grossmann, F.; Kamenya, S.; Pintea, L.; et al. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog. 2010, 6, e1001116. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; Vigilant, L. A population estimate of chimpanzees (Pan troglodytes schweinfurthii) in the Ugalla region using standard and spatially explicit genetic capture—recapture methods. Am. J. Primatol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Plumptre, A.J.; Rose, R.; Nangendo, G.; Williamson, E.A.; Didier, K.; Hart, J.; Mulindahabi, F.; Hicks, C.; Griffin, B.; Ogawa, H.; et al. Eastern Chimpanzee (Pan troglodytes schweinfurthii) Status Survey and Conservation Action Plan 2010–2020; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2010. [Google Scholar]
- Piel, A.K.; Stewart, F.A. Census and Conservation Status of Chimpanzees (Pan troglodytes schweinfurthii) Across the Greater Mahale Ecosystem; The Nature Conservancy: Arlington, VA, USA, 2014; 74p. [Google Scholar]
- Kano, T.; Ogawa, H.; Asato, R.; Kanamori, M. Distribution and density of wild chimpanzees on the northwestern bank of the Malagarasi River, Tanzania. Primate Res. 1999, 15, 153–162. [Google Scholar] [CrossRef]
- Ogawa, H.; Yoshikawa, M.; Mbalamwezi, M. A Chimpanzee bed found at Tubila, 20 km from Lilanshimba habitat. Pan Africa News 2011, 18, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Zamma, K.; Inoue, E. On the chimpanzees of Kakungu, Karobwa and Ntakata. Pan Africa News 2004, 11, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Plumptre, A.J.; Cox, D. Counting primates for conservation: Primate surveys in Uganda. Primates 2006, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.D.; Williams, B.K. Monitoring for conservation. Trends Ecol. Evol. 2006, 21, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.; Jácomo, A.T.A; Diniz-Filho, J.A.F. Camera trap, line transect census and track surveys: A comparative evaluation. Biol. Conserv. 2003, 114, 351–355. [Google Scholar] [CrossRef]
- Piel, A.K.; Cohen, N.; Kamenya, S.; Ndimuligo, S.A.; Pintea, L.; Stewart, F.A. Population status of chimpanzees in the Masito-Ugalla Ecosystem, Tanzania. Am. J. Primatol. 2015, 77, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Wich, S.A.; Singleton, I.; Nowak, M.G.; Utami Atmoko, S.S.; Nisam, G.; Arif, S.M.; Putra, R.H.; Ardi, R.; Fredriksson, G.; Usher, G.; et al. Land-cover changes predict steep declines for the Sumatran orangutan (Pongo abelii). Sci. Adv. 2016, 2, e1500789. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.J.; Strindberg, S.; Bakabana, P.C.; Elkan, P.W.; Iyenguet, F.C.; Madzoké, B.; Malanda, G.A.F.; Mowawa, B.S.; Moukoumbou, C.; Ouakabadio, F.K.; et al. Monitoring great ape and elephant abundance at large spatial scales: Measuring effectiveness of a conservation landscape. PLoS ONE 2010, 5, e10294. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, C.Y.; Boesch, C.; Kuehl, H. Estimating chimpanzee population size with nest counts: Validating methods in Ta? National Park. Am. J. Primatol. 2009, 71, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Spehar, S.N.; Mathewson, P.D.; Nuzuar; Wich, S.A.; Marshall, A.J.; Kühl, H.; Nardiyono; Meijaard, E. Estimating orangutan densities using the standing crop and marked nest count methods: Lessons learned for conservation. Biotropica 2010, 42, 748–757. [Google Scholar] [CrossRef]
- Kidney, D.; Rawson, B.M.; Borchers, D.L.; Stevenson, B.C.; Marques, T.A.; Thomas, L. An efficient acoustic density estimation method with human detectors applied to gibbons in Cambodia. PLoS ONE 2016, 11, e155066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Kühl, H.; Maisels, F.; Ancrenaz, M.; Williamson, E.A. Best Practice Guidelines for Surveys and Monitoring of Great Ape Populations; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2009. [Google Scholar]
- Jachmann, H. Comparison of aerial counts with ground counts for large African herbivores. J. Appl. Ecol. 2002, 39, 841–852. [Google Scholar] [CrossRef]
- Kirkman, S.P.; Yemane, D.; Oosthuizen, W.H.; Meÿer, M.A.; Kotze, P.G.H.; Skrypzeck, H.; Vaz Velho, F.; Underhill, L.G. Spatio-temporal shifts of the dynamic Cape fur seal population in Southern Africa, based on aerial censuses (1972–2009). Mar. Mammal Sci. 2013, 29, 497–524. [Google Scholar] [CrossRef]
- Greene, K.; Bell, D.; Kioko, J.; Kiffner, C. Performance of ground-based and aerial survey methods for monitoring wildlife assemblages in a conservation area of northern Tanzania. Eur. J. Wildl. Res. 2017, 63, 77. [Google Scholar] [CrossRef]
- Sasse, D.B. Job-related mortality of wildlife workers in the United States, 1937–2000. Wildl. Soc. Bull. 2003, 31, 1000–1003. [Google Scholar]
- Yang, Z.; Wang, T.; Skidmore, A.K.; De Leeuw, J.; Said, M.Y.; Freer, J. Spotting East African mammals in open savannah from space. PLoS ONE 2014, 9, e115989. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Roy, D.P.; Lindquist, E.; Adusei, B.; Justice, C.O.; Altstatt, A. A method for integrating MODIS and Landsat data for systematic monitoring of forest cover and change in the Congo Basin. Remote Sens. Environ. 2008, 112, 2495–2513. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Carbone, C. Surveys using camera traps: Are we looking to a brighter future? Anim. Conserv. 2008, 11, 185–186. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Mennill, D.J.; Clemins, P.; Girod, L.; Yao, K.; Patricelli, G.; Deppe, J.L.; Krakauer, A.H.; Clark, C.; Cortopassi, K.A.; et al. Acoustic monitoring in terrestrial environments using microphone arrays: Applications, technological considerations and prospectus. J. Appl. Ecol. 2011, 48, 758–767. [Google Scholar] [CrossRef]
- Koh, L.P.; Wich, S.A. Dawn of drone ecology: low-cost autonomous aerial vehicles for conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Chabot, D.; Bird, D.M. Wildlife research and management methods in the 21st century: Where do unmanned aircraft fit in? J. Unmanned Veh. Syst. 2015, 3, 137–155. [Google Scholar] [CrossRef]
- Wich, S.A. Drones and conservation. In Drones and Aerial Observation: New Technologies for Property Rights, Human Rights, and Global Development. A Primer; Kakaes, K., Ed.; New America: Washington, DC, USA, 2015; pp. 63–71. [Google Scholar]
- Chabot, D.; Carignan, V.; Bird, D.M. Measuring habitat quality for least bitterns in a created wetland with use of a small unmanned aircraft. Wetlands 2014, 34, 527–533. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Stolper, R.; Van Essen, L.D.; Negro, J.J.; Sassen, T. Remotely piloted aircraft systems as a rhinoceros anti-poaching tool in Africa. PLoS ONE 2014, 9, e83873. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.; Lejeune, P.; Lisein, J.; Sawadogo, P.; Bouché, P. Unmanned aerial survey of elephants. PLoS ONE 2013, 8, e54700. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.J.; Kelly, N.; Peel, D. Unmanned aerial vehicles (UAVs) for surveying Marine Fauna: A dugong case study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef] [PubMed]
- Koski, W.R.; Allen, T.; Ireland, D.; Buck, G.; Smith, P.R.; Macrender, A.M.; Halick, M.A.; Rushing, C.; Sliwa, D.J.; McDonald, T.L. Evaluation of an unmanned airborne system for monitoring marine mammals. Aquat. Mamm. 2009, 35, 347–357. [Google Scholar] [CrossRef]
- Koski, W.R.; Gamage, G.; Davis, A.R.; Mathews, T.; LeBlanc, B.; Ferguson, S.H. Evaluation of UAS for photographic re-identification of bowhead whales, Balaena mysticetus. J. Unmanned Veh. Syst. 2015, 3, 22–29. [Google Scholar] [CrossRef]
- Hodgson, A.; Peel, D.; Kelly, N. Unmanned aerial vehicles for surveying marine fauna: Assessing detection probability. Ecol. Appl. 2017, 27, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Wich, S.A.; Dellatore, D.; Houghton, M.; Ardi, R.; Koh, L.P. A preliminary assessment of using conservation drones for Sumatran orang-utan (Pongo abelii) distribution and density. J. Unmanned Veh. Syst. 2015, 4, 45–52. [Google Scholar] [CrossRef]
- Van Andel, A.C.; Wich, S.A.; Boesch, C.; Koh, L.P.; Robbins, M.M.; Kelly, J.; Kuehl, H.S. Locating chimpanzee nests and identifying fruiting trees with an unmanned aerial vehicle. Am. J. Primatol. 2015, 77, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.H.; Kendall, W.L. Visibility bias in aerial surveys: A review of estimation procedures. J. Wildl. Manag. 1987, 51, 502–510. [Google Scholar] [CrossRef]
- Buckland, S.; Anderson, D.R.; Burnham, K.; Laake, J.; Borchers, D.; Thomas, L. Advanced Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Dulava, S.; Bean, W.T.; Richmond, O.M.W. Environmental reviews and case studies: Applications of unmanned aircraft systems (UAS) for waterbird surveys. Environ. Pract. 2015, 17, 201–210. [Google Scholar] [CrossRef]
- Patterson, C.; Koski, W.; Pace, P.; Mcluckie, B.; Bird, D.M. Evaluation of an unmanned aircraft system for detecting surrogate caribou targets in Labrador. J. Unmanned Veh. Syst. 2016, 4, 53–69. [Google Scholar] [CrossRef]
- Moore, J. Savanna chimpanzees. In Topics in Primatology, Vol.1 Human Origins; Nishida, T., McGrew, P., Marler, P., Pickford, M., de Waal, F., Eds.; University of Tokyo Press: Tokyo, Japan, 1992; pp. 99–118. [Google Scholar]
- Anokwa, Y.; Hartung, C.; Brunette, W.; Borriello, G.; Lerer, A. Open source data collection in the developing world. Computer 2009, 42. [Google Scholar] [CrossRef]
- Linchant, J.; Lhoest, S.; Quevauvillers, S.; Semeki, J.; Lejeune, P.; Vermeulen, C. WIMUAS: Developing a tool to review wildlife data from various UAS flight plans. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. ISPRS Arch. 2015, 40, 379–384. [Google Scholar] [CrossRef]
- Macmillan, N.A.; Creelman, C.D. Detection Theory: A User’s Guide; Laurence Erlbaum Associates Inc.: Mahwah, NJ, USA, 2005. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 2014, 1, 1–23. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- López-Bao, J.V.; Rodríguez, A.; Palomares, F. Behavioural response of a trophic specialist, the Iberian lynx, to supplementary food: Patterns of food use and implications for conservation. Biol. Conserv. 2008, 141, 1857–1867. [Google Scholar] [CrossRef]
- Ancrenaz, M.; Gimenez, O.; Ambu, L.; Ancrenaz, K.; Andau, P.; Goossens, B.; Payne, J.; Sawang, A.; Tuuga, A.; Lackman-Ancrenaz, I. Aerial surveys give new estimates for orangutans in Sabah, Malaysia. PLoS Biol. 2005, 3, e30003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Casteren, A.; Sellers, W.I.; Thorpe, S.K.S.; Coward, S.; Crompton, R.H.; Myatt, J.P.; Ennos, A.R. Nest-building orangutans demonstrate engineering know-how to produce safe, comfortable beds. Proc. Natl. Acad. Sci. USA 2012, 109, 6873–6877. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.T.; Gerard, P.D.; Dinsmore, S.J.; Kaminski, R.M.; Reinecke, K.J. Estimation and correction of visibility bias in aerial surveys of wintering ducks. J. Wildl. Manag. 2008, 72, 808–813. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Evaluation of an off-the-shelf unmanned aircraft system for surveying flocks of geese. Waterbirds 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Stewart, F.A. The Evolution of Shelter: Ecology and Ethology of Chimpanzee Nest Building; University of Cambridge: Cambridge, UK, 2011. [Google Scholar]
- Hicks, T.C. A Chimpanzee Mega-Culture? Exploring Behavioral Continuity in Pan Troglodytes Schweinfurthii Across Northern DR Congo. Ph.D. Dissertation, Universiteit Van Amsterdam, Amsterdam, The Netherland, 2010. [Google Scholar]
- Van Schaik, C.P.; Wich, S.A.; Utami, S.S.; Odom, K. A simple alternative to line transects of nests for estimating orangutan densities. Primates 2005, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.F.; Montes, G.A.; Puig, E.; Johnson, S.; Mengersen, K.; Gaston, K.J. Unmanned aerial vehicles (UAVs) and artificial intelligence revolutionizing wildlife monitoring and conservation. Sensors 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selby, W.; Corke, P.; Rus, D. Autonomous aerial navigation and tracking of marine animals. In Proceedings of the Australasian Conference on Robotics and Automation, Melbourne, Australia, 7–9 December 2011; pp. 1–7. [Google Scholar]
- Abd-Elrahman, A.; Pearlstine, L.; Percival, F. Development of pattern recognition algorithm for automatic bird. Surv. Land Inf. Sci. 2005, 65, 37. [Google Scholar]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Segaran, R.R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 1, 1–19. [Google Scholar] [CrossRef]
- Andrew, M.E.; Shephard, J.M. Semi-automated detection of eagle nests: An application of very high-resolution image data and advanced image analyses to wildlife surveys. Remote Sens. Ecol. Conserv. 2017, 3, 66–80. [Google Scholar] [CrossRef]
- Duffy, J.P.; Anderson, K. A 21st-century renaissance of kites as platforms for proximal sensing. Prog. Phys. Geogr. Earth Environ. 2016, 40, 352–361. [Google Scholar] [CrossRef]
- Du, T.; Schulz, A.; Csail, M.; Zhu, B.; Bickel, B.; Matusik, W. Computational multicopter design. ACM Trans. Graph. 2016, 35. [Google Scholar] [CrossRef]
- Magnussen, Ø.; Hovland, G.; Ottestad, M. Multicopter UAV design optimization. In Proceedings of the 2014 IEEE/ASME 10th International Conference on Mechatronic and Embedded Systems and Applications (MESA), Senigallia, Italy, 10–12 September 2014. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Member, S.; Zarco-tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Gini, R.; Passoni, D.; Pinto, L.; Sona, G. Use of unmanned aerial systems for multispectral survey and tree classification: A test in a park area of northern Italy. Eur. J. Remote Sens. 2014, 47, 251–269. [Google Scholar] [CrossRef]
- Woll, C.; Prakash, A.; Sutton, T. A case-study of in-stream juvenile salmon habitat classification using decision-based fusion of multispectral aerial images. Appl. Remote Sens. 2011, 2, 37–46. [Google Scholar]
- Sugiura, R.; Noguchi, N.; Ishii, K. Remote-sensing technology for vegetation monitoring using an unmanned helicopter. Biosyst. Eng. 2005, 90, 369–379. [Google Scholar] [CrossRef]
- Arnold, T.; De Biasio, M.; Fritz, A.; Leitner, R. UAV-based measurement of vegetation indices for environmental monitoring. In Proceedings of the 2013 7th International Conference on Sensing Technology, ICST, Wellington, New Zealand, 3–5 December 2013; pp. 704–707. [Google Scholar]
- De Biasio, M.; Arnold, T.; Leitner, R.; McGunnigle, G.; Meester, R. UAV-based environmental monitoring using multi-spectral imaging. Proc. SPIE 2010, 766811. [Google Scholar] [CrossRef]
- Greenwood, F. How to make maps with drones. In Drones and Aerial Observation: New Technologies for Property Rights, Human Rights, and Global Development; New America: Washington, DC, USA, 2015; pp. 35–47. [Google Scholar]
- Wallace, L.; Lucieer, A.; Watson, C.; Turner, D. Development of a UAV-LiDAR system with application to forest inventory. Remote Sens. 2012, 4, 1519–1543. [Google Scholar] [CrossRef]
- Gooday, O.J.; Key, N.; Goldstien, S.; Zawar-Reza, P. An assessment of thermal-image acquisition with an unmanned aerial vehicle (UAV) for direct counts of coastal marine mammals ashore. J. Unmanned Veh. Syst. 2018. [Google Scholar] [CrossRef]
- Hicks, T.C.; Tranquilli, S.; Kuehl, H.; Campbell, G.; Swinkels, J.; Darby, L.; Boesch, C.; Hart, J.; Menken, S.B.J. Absence of evidence is not evidence of absence: Discovery of a large, continuous population of Pan troglodytes schweinfurthii in the Central Uele region of northern DRC. Biol. Conserv. 2014, 171, 107–113. [Google Scholar] [CrossRef]


| Predictors | LRT | Parameter Estimate | ||||
|---|---|---|---|---|---|---|
| Χ2 | p Value | Estimate | Std. E. | z Value | Pr (>|z|) | |
| (Intercept) | −2.96 | 0.59 | −5.01 | 5.66 × 10−7 | ||
| Drone/camera pairing (Pairing A) | 10.96 | 0.004 ** | ||||
| Pairing B | 1.43 | 0.57 | −2.49 | 0.013 * | ||
| Vegetation (closed) | 0.89 | 0.828 | ||||
| Open | 0.3 | 0.84 | 0.37 | 0.722 | ||
| Season (May 2015) | 0.40 | 0.818 | ||||
| Sep-16 | −0.35 | 0.78 | −0.45 | 0.651 | ||
| Drone/camera pairing: Vegetation | 0.55 | 0.457 | ||||
| Pairing A: Open vegetation | 0.57 | 0.76 | 0.74 | 0.458 | ||
| Vegetation: Season | 7.29 | 0.993 | ||||
| Open vegetation: September 2016 | 0.01 | 1 | 0.01 | 0.993 | ||
| Predictors | LRT | Parameter Estimate | ||||
|---|---|---|---|---|---|---|
| Χ2 | p Value | Estimate | Std. E. | z Value | Pr (>|z|) | |
| (Intercept) | −1.53 | 0.28 | −5.45 | 4.98 × 10−8 | ||
| Flight altitude AGL | 4.35 | 0.037 * | −0.47 | 0.25 | −1.90 | 0.057 |
| Vegetation (closed) | 2.79 | 0.094 | ||||
| Open | −0.68 | 0.40 | −1.70 | 0.089 | ||
| Nest height | 0.07 | 0.789 | 0.04 | 0.17 | 0.27 | 0.789 |
| Predictors | LRT | Parameter Estimate | ||||
|---|---|---|---|---|---|---|
| Χ2 | p Value | Estimate | Std. E. | z Value | Pr (>|z|) | |
| (Intercept) | −3.03 | 1.19 | −2.54 | 0.011 * | ||
| Drone/camera pairing (Pairing A) | 14.14 | 1.17 × 10−4 *** | ||||
| Pairing B | 3.69 | 1.08 | 3.40 | 6.73 × 10−4 *** | ||
| Vegetation (closed) | 23.23 | 1.44 × 10−6 *** | ||||
| Open | 5.72 | 1.99 | 2.87 | 0.004 ** | ||
| Season (May 2015) | 0.04 | 0.834 | ||||
| Sep-16 | 2.86 | 1.16 | 2.47 | 0.013 * | ||
| Flight altitude AGL | 9.55 | 0.002 ** | 2.01 | 0.90 | 2.24 | 0.025 * |
| Drone/camera pairing: Vegetation | 0.05 | 0.824 | ||||
| Pairing A: Open vegetation | −3.72 | 1.56 | −2.38 | 0.017 * | ||
| Season: Vegetation | 4.01 | 0.045 * | ||||
| Sept 2016: Open vegetation | −7.27 | 1.83 | −3.98 | 6.83 × 10−5 *** | ||
| Vegetation: Flight altitude AGL | 0.37 | 0.542 | ||||
| Open vegetation: Flight altitude AGL | −5.98 | 1.63 | −3.67 | 2.40 × 10−4 *** | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnin, N.; Van Andel, A.C.; Kerby, J.T.; Piel, A.K.; Pintea, L.; Wich, S.A. Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images. Drones 2018, 2, 17. https://doi.org/10.3390/drones2020017
Bonnin N, Van Andel AC, Kerby JT, Piel AK, Pintea L, Wich SA. Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images. Drones. 2018; 2(2):17. https://doi.org/10.3390/drones2020017
Chicago/Turabian StyleBonnin, Noémie, Alexander C. Van Andel, Jeffrey T. Kerby, Alex K. Piel, Lilian Pintea, and Serge A. Wich. 2018. "Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images" Drones 2, no. 2: 17. https://doi.org/10.3390/drones2020017
APA StyleBonnin, N., Van Andel, A. C., Kerby, J. T., Piel, A. K., Pintea, L., & Wich, S. A. (2018). Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images. Drones, 2(2), 17. https://doi.org/10.3390/drones2020017






