1. Introduction
The eyes are an important connection between the outer and inner worlds. In Ayurveda (an Indian system of medicine), pitta dosha stands for the element of fire and light that governs our eyes. Hence, the eyes are very important organs in our body. For care and beautification of the eyes, Vedic science offers several natural, safe, and effective techniques. With the help of Ayurvedic science, several herbs and floras have been used to make Ayurvedic cosmetics that not only beautify the skin but also protect against external effects on the body. In cosmetics, for useful purposes such as moisturizing, whitening, coloring, sunscreens, antioxidants, immunostimulants, cleansing, preservatives, thickeners, etc., plant products are also used. The role of kajal in eye products cannot be ignored, as it is one of those products [
1].
Kajal is worn for many reasons, including tradition, beautification, or to ward off the “evil eye”. It is a widespread belief that kohl is medically beneficial for the eyes, and wearing kohl is encouraged in the Sunna, which encompasses the traditional behavioral guidelines of the Islamic religion [
2]. There are a number of plants which are used for ophthalmic disorders, either aloneorin compound formulations, that can be found in the Ayurvedic system of medicine, as mentioned in ancient Indian books such as
Charak Samhita,
Sushrut Samhita,
Bhav Prakasha,
Ras Tarang,
Nayan Drastam, and
Astanghriday. Various eye disorders and diseases, such as
Abhishyand (conjunctivitis),
Adhimanth (glaucoma),
Timir (cataract), etc., have been described in great detailin Ayurveda. Their etiology and treatments have also been described. The use of various herbal drugs in different dosage forms, such as extracts,
arkas (aqueous distillate),
kajal (collerium), and fomentation and washing with different extracts, has also been prescribed frequently [
3]. Not only the use of animals for laboratory testing but also the materials and ingredients derived from animal sources is an concern of area regarding the standards and quality of drugs and cosmetics manufactured and sold in India, which are governed by the Drugs and Cosmetics Act [
1].
Hence, for the “natural” products used in various preparations, there is a need for regulation. The best way to create trust and confidence in these products and increase market relevance is through standardization. The consumer as well as the practitioner now seeks assurance from the manufacturer about the quality, safety, and efficacy of products. Thus, earlier recommendations related to cosmetic preparations with herbs for specific conditions may not hold true today unless properly validated [
4]. In this study, different extracts of
Rosa rubiginosa and Triphala herbs were selected to formulate kajal. Well known in Ayurveda, Triphala is a polyherbal formulation that is a Rasayana drug used in the Indian System of Medicine (ISM) [
5]. As described in the Ayurvedic formulary of India, it is a mixture of the dried fruits of
Emblica officinalis Gaertn (Euphorbiaceae),
Terminalia bellerica Linn (Combertaceae), and
Terminalia chebula (Combertaceae) inequal proportions (1:1:1) [
6]. The most commonly found polyphenolic compounds in plant extracts are tannins, gallic acid, chebulagic acid, ellagic acid, phenols, and glycosides. Phenolic acids, flavonoids, and tannins are commonly found in Triphala [
7]. Triphala is also widely taken for many eye diseases, including the treatment of conjunctivitis, progressive myopia, the early stages of glaucoma, and cataracts, as described by Mahajan et al. Triphala ghrita is given at a dose of 1080 mg to delay the onset and progression of cataracts. The antioxidant activity of gallic acid, ellagic acid, and ascorbic acid may also have anticataract effects [
8]. For the cure of constipation, inflammation, and swelling and to ease throat infections, rose water has traditionally been used. However, when used as eye drops, it can help cure eye issues. Herbal preparations that also include rose extracts have been used to prepare eye drops for use on patients suffering from dry eyes, conjunctivitis, and pterygium. Such eye drops are also used by postoperative cataract patients, and a significant improvement in their condition has been noticed. Needless to say, rose water is good for the eyes.
Rose water helps to soothe the eyes, treat skin irritation, prevent cell damage, and lighten dark circles [
9]. Formulating medicated kajal for beautification and as a cosmeceutical product to combat eye infections has been thought of as an innovative approach, as kajal’s medicinal use is limited and it is mostly used as eye makeup. Considering this, the present study aimed to prepare a contemporary formulation from the preliminary Ayurvedic kajal called soot/lamp black using two herbs, namely,
R. rubiginosa and Triphala and to standardize it in terms of identity, physical evaluation, and toxicity.
2. Materials and Methods
2.1. Collection of Plant Materials andProximal Analysis
The fresh fruits of Triphala, such as amala, baheda, and haritaki, and the flowers of
R. rubiginosa were collected. Quantitative standards for the fruits of Triphala and the petals of the
R. rubiginosa flower, in terms of moisture content, total ash, acid-insoluble ash, and alcohol- and water-soluble extractive values for both samples, were performed by preliminary macroscopical identification of both the raw plant materials. This was carried out as per the method described by the World Health Organization (WHO) guidelines [
10].
2.2. Preliminary Ayurvedic Formulation of Kajal
| The required quantity of rose petals and the dried powder of Triphala fruits for preparing the extract was acquired. |
![Proceedings 09 00069 i001 Proceedings 09 00069 i001]() |
| The extract was hygienically prepared, and an unbleached cloth was soaked in the rose extract and dried in a hot air oven. |
![Proceedings 09 00069 i001 Proceedings 09 00069 i001]() |
| The dried cloth piece was used as a wick and was lighted in a mud lamp containing cow ghee. |
![Proceedings 09 00069 i001 Proceedings 09 00069 i001]() |
| The black soot was collected in a clean, dry porcelain dish. |
![Proceedings 09 00069 i001 Proceedings 09 00069 i001]() |
| The powder was then mixed with cow ghee to form a paste (i.e., kajal). |
2.3. Contemporary Formulation of Kajal
The modern kajal (
Table 1) was formulated in the same manner as preliminary Ayurvedic kajal,andthe ingredients are given below in
Table 1.
2.4. Standardization of Ingredients, Processes,and Products
The standardization of the quality of the ingredients used in theproduct was done by using thin-layer chromatography (TLC). The physiochemical characteristics of the lamp black, plant extracts of two separate herb plants and the kajal powder were determined by the Karl Fischer method (for moisture content), a penetrometer test (the pressure at which the needle penetrates), TLC, and microbial contamination and toxicity studies.
2.4.1. TLC of Extracts
Both the
R. rubiginosa and Triphala extracts were checked by TLC (
Table 2 and
Table 3) on analytical plates over silica gel. The isolation of the principle components that were present in most effective extracts of the plants and the final products were carried out using TLC. The different polarities of the solvents were prepared, and for better resolution, TLC studies were carried out to select a suitable solvent system [
11]. Based on that, 6 g of the product (prepared from
R. rubiginosa) was subjected to Soxhlet extraction with water as a solvent. Further, 65 g of whole-plant Triphala was refluxed with 100 mL of alcohol for 1 h and then evaporated in awater bath to reduce the volume. The crude extract was then diluted to a sufficient volume of alcohol.
Finally, the leaf extract of R. rubiginosa, lamp black, and kajal products and the alcoholic plant extract of Triphala, lamp black, and kajal products were subjected to TLC using two separate mobile phases, viz., toluene:acetone:formic acid (11:6:1) and toluene:ethyl acetate (95:5) for R. rubiginosa and Triphala, respectively. Both of the samples were visualized at 254 and 366 nm, and a band of similar Rf values was identified.
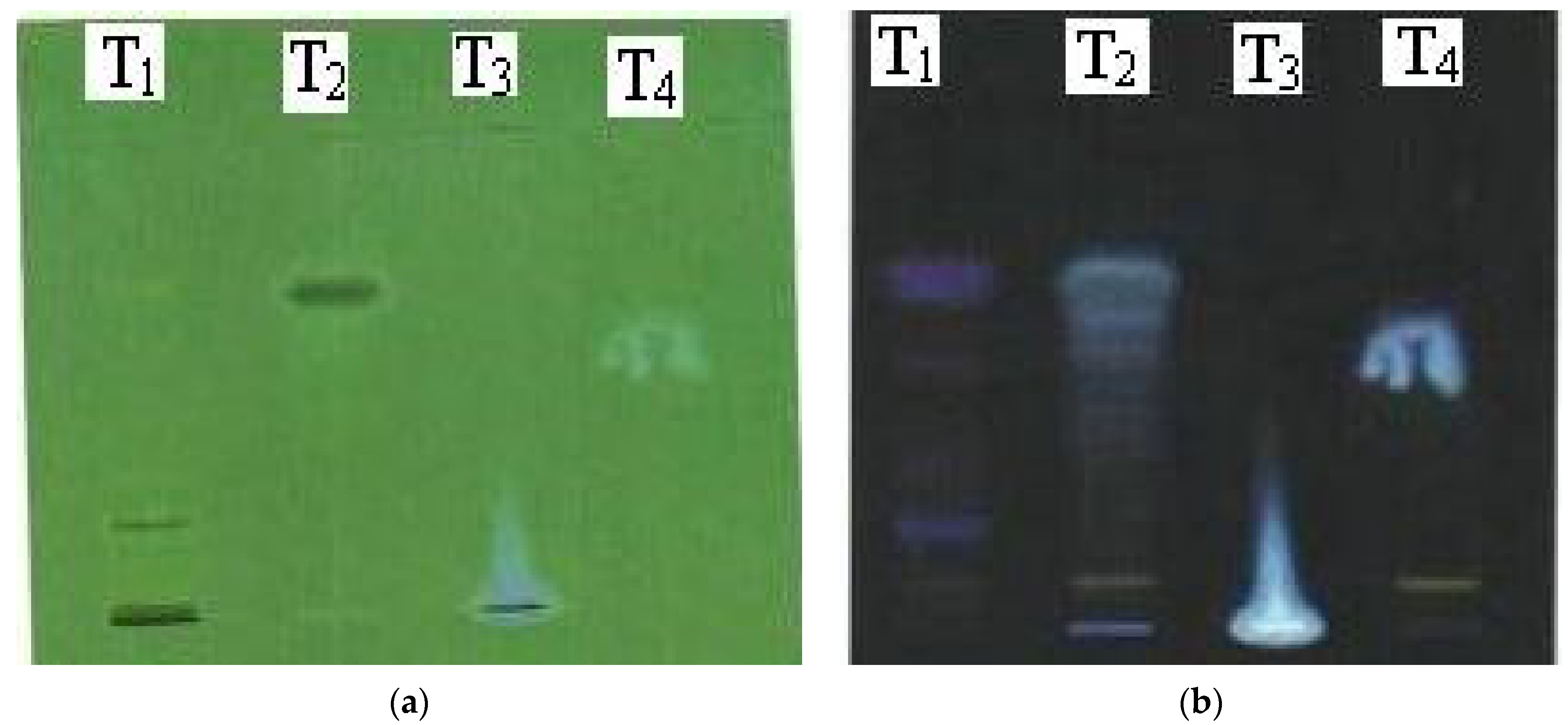
Figure 1.
TLC plates at different wavelengths for R. rubiginosakajal extracts: (a) UV at 254 nm; (b) UV at 366 nm.
Figure 1.
TLC plates at different wavelengths for R. rubiginosakajal extracts: (a) UV at 254 nm; (b) UV at 366 nm.
2.4.2. Microbial Contamination
Microbial tests were performed to check the quality of the kajal products to determine the microbial, total fungal, and Escherichia coli countsby acylindrical plate method. Nutrient agar (for total bacterial count), MacConkey agar (for total E. coli count), and Sabouraud dextrose agar (for fungal and yeast count) were incubated at 37 °C overnight, 43 °C overnight, and 25 °C for 4 days, respectively. Finally, the growth was observed and, by a colony counter, counted in per volume plated, and the results are expressed as CFU/g (colony forming units per gram of the sample).
2.5. Evaluation of Allergenic Studies on Kajal Products
Physical Evaluation: The formulated product was of a shiny black color, with a characteristic odor. It was non gritty and smooth in texture with a semisolid consistency.
PH Determination: From a pH meter; read as 7.2
Viscosity Determinations: By using a Brookfield Viscometer.
Evaluation of Base: For acid, saponification, and ester values, vegetable ghee was evaluated as per I.P. 1996.
Acid Value: Acid value = 5.61 × n/w, where n is the number of ML of 0.1 M KOH required and w is the weight in grams of the solvent; n = 0.5 and w = 2.0. Thus, the acid value was 5.61 × 0.5/2 = 1.4025.
Saponification Value: Saponification value = 28.05 (b − a)/w, where w is the weight in grams of the substance, b is the blank solution reading, and a is the sample solution reading; b = 21, a = 4.6, and w = 2.0 Therefore, the saponification value was 28.05 (21 − 4.6)/2 = 230.01.Theester value equals the saponification value minus the acid value. Thus, ester value was230.01 − 1.4025 = 228.6075.
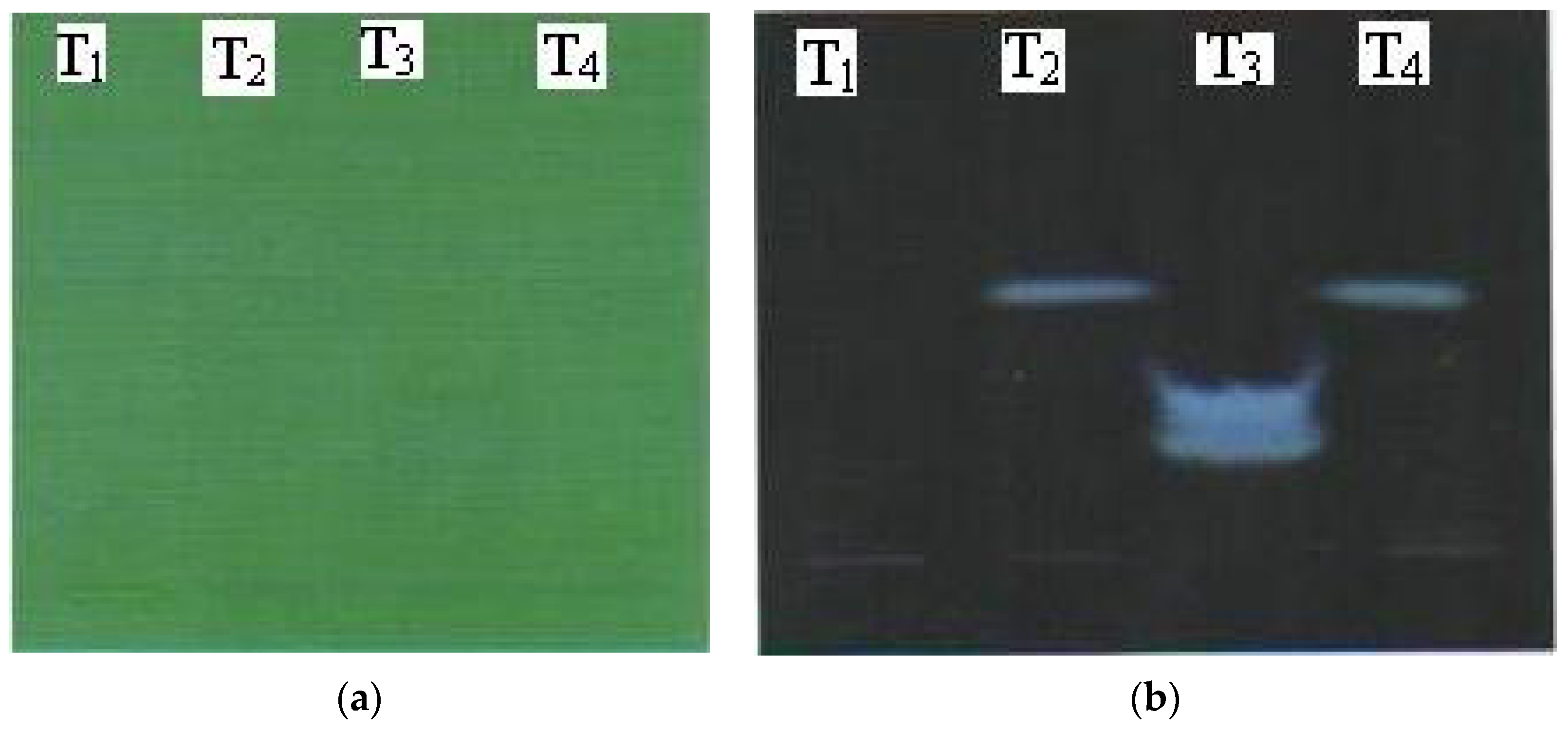
Figure 2.
TLC plates at different wavelengths for Triphala kajal extracts: (a) UV at 254 nm; (b) UV at 366 nm.
Figure 2.
TLC plates at different wavelengths for Triphala kajal extracts: (a) UV at 254 nm; (b) UV at 366 nm.