Stereoselective Hydrostannation of Diacrylate and Dimethacrylate Esters of Galactaric Acid Derivatives: Cyclohydrostannation vs. Diaddition †
Abstract
:1. Introduction
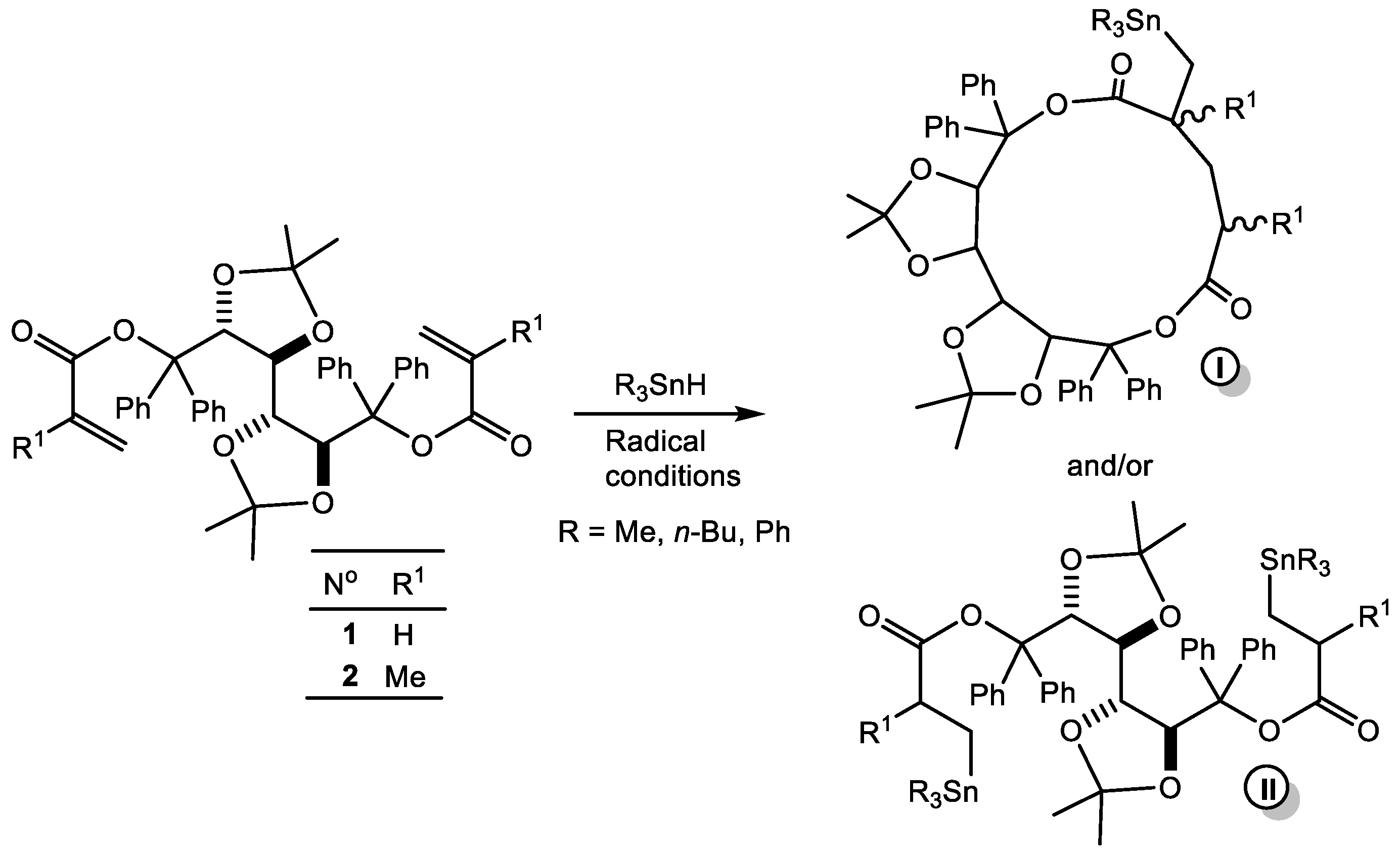
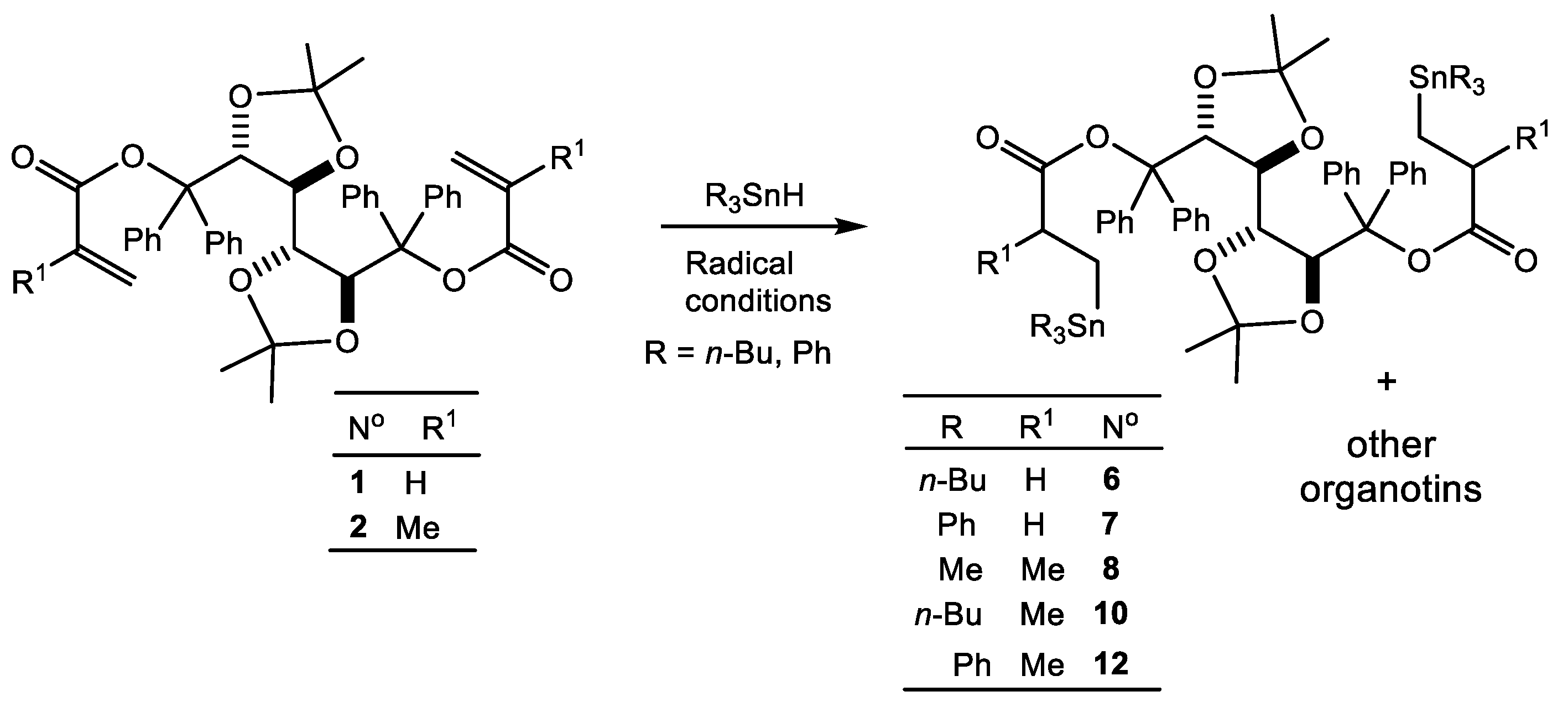
2. Results and Discussion
Acknowledgments
Conflicts of Interest
References
- Gerbino, D.C.; Koll, L.C.; Mandolesi, S.D.; Podestá, J.C. Stereoselective radical tandem cyclohydrostannation of optically active di-unsaturated esters of TADDOL. Organometallics 2008, 27, 660–665. [Google Scholar] [CrossRef]
- Gerbino, D.C.; Scoccia, J.; Koll, L.C.; Mandolesi, S.D.; Podestá, J.C. Stereoselective Synthesis and Some Properties of New Chlorodiorganotin-Substituted Macrodiolides. Organometallics 2012, 31, 662–671. [Google Scholar] [CrossRef]
- Scoccia, J.; Gerbino, D.C.; Podestá, J.C. Synthesis of organotin derivatives of optically active eleven-membered macrodiolides. Tetrahedron Asymmetry 2016, 27, 352–360. [Google Scholar] [CrossRef]
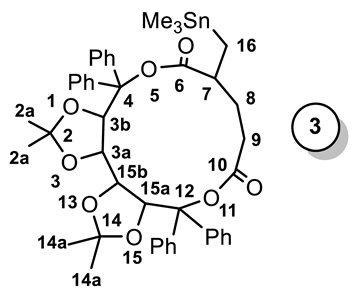 | 13C NMR a (ppm) (CDCl3) | Me–Sn | C-6 | C-7 | C-8 | C-10 | C-16 |
| −8.75 (33.4) | 172.99 (39.9) | 53.58 (NO) | 31.73 (41.0) | 171.63 | 15.33 (341.4) | ||
| 1H NMR (ppm) (CDCl3) | −0.08 (d, 2H); 0.00 [s, 9H, 2J(Sn, H) = 52.4 Hz]; 0.68–1.06 (m, 3H); 1.19 (s, 6H); 1.28 (s, 6H); 1.87–2.35 (m, 1H); 3.25–3.50 (m, 1H); 4.75–4.94 (m, 1H); 4.99–5.39 (m, 1H); 5.89 (s, 2H); 6.90–7.70 (m, 20H). | ||||||
| 119Sn NMR (ppm) (CDCl3) | −3.34 | ||||||
| FTIR (cm−1) (Nujol) | 1760.05; 1732.63. | ||||||
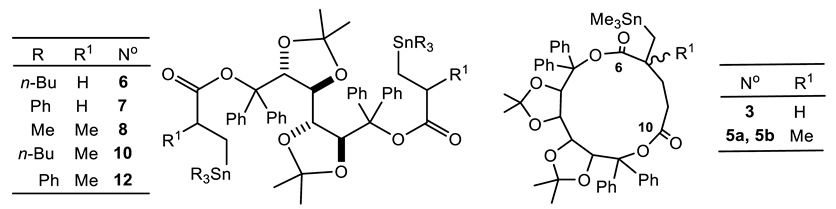
| Reaction N° | R | Ester N° | Time (h) | Adduct N° b | 119Sn NMR (ppm) c | % in the Mixture | 13C NMR C=O (ppm) | |
|---|---|---|---|---|---|---|---|---|
| C-6 | C-10 | |||||||
| Me | Me | 1 | 3 4a 4b | −3.34 −0.1 5.6 | 48 20 32 | 171.63 | 172.99 | |
| 2 | Me | 2 | 16 | 5a 5b | −0.8 1.5 | 73 27 | 164.88 164.93 | 175.29 175.39 |
| 3 d | n-Bu | 1 | 1 | 6a 7 | −8.0 −7.8 | 9 91 | 173.04 (64.5) | |
| 4 | Ph | 1 | 3 | 8 | −98.2 | 100 | 172.56 (77.2) | |
| 5 | n-Bu | 2 | 1 | 9 10a 10b | −12.0 −9.9 −9.6 | 60 20 20 | 174.84 175.17 175.30 175.57 | |
| 6 | Ph | 2 | 3 | 11 12a 12b | −104.0 −101.0 −100.8 | 75 12.5 12.5 | 174.73 174.88 175.03 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terraza, V.F.; Gerbino, D.C.; Podestá, J.C. Stereoselective Hydrostannation of Diacrylate and Dimethacrylate Esters of Galactaric Acid Derivatives: Cyclohydrostannation vs. Diaddition. Proceedings 2019, 9, 55. https://doi.org/10.3390/ecsoc-22-05688
Terraza VF, Gerbino DC, Podestá JC. Stereoselective Hydrostannation of Diacrylate and Dimethacrylate Esters of Galactaric Acid Derivatives: Cyclohydrostannation vs. Diaddition. Proceedings. 2019; 9(1):55. https://doi.org/10.3390/ecsoc-22-05688
Chicago/Turabian StyleTerraza, V. Fabricio, Darío C. Gerbino, and Julio C. Podestá. 2019. "Stereoselective Hydrostannation of Diacrylate and Dimethacrylate Esters of Galactaric Acid Derivatives: Cyclohydrostannation vs. Diaddition" Proceedings 9, no. 1: 55. https://doi.org/10.3390/ecsoc-22-05688
APA StyleTerraza, V. F., Gerbino, D. C., & Podestá, J. C. (2019). Stereoselective Hydrostannation of Diacrylate and Dimethacrylate Esters of Galactaric Acid Derivatives: Cyclohydrostannation vs. Diaddition. Proceedings, 9(1), 55. https://doi.org/10.3390/ecsoc-22-05688




