The Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazol-4-carbonitrile with beta-Cycloketols †
Abstract
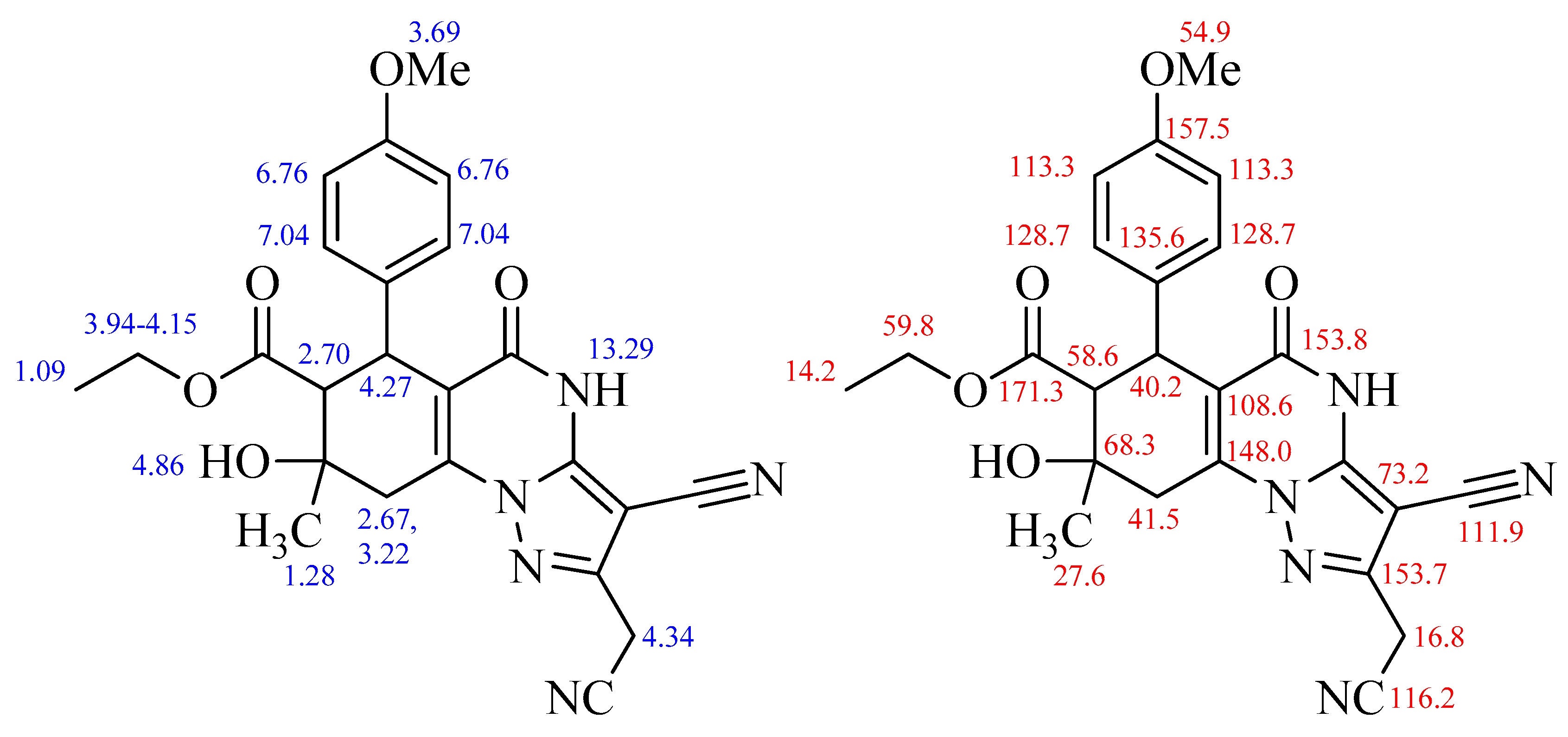
:Experimental
References
- Kriven’ko, A.P.; Sorokin, V.V. Substituted cyclohexanolons. Saratov, 1999. P. 20 (In Russian).
- Ismiev, A.I.; Maharramov, A.M.; Sukach, V.A.; Vovk, M.V. (Synthesis and reactions of diacetyl(dialkoxycarbonyl)-substituted hydroxycyclohexanones) J. Org. Pharm. Chem. 2016, 14, 16. (In Russian). Available online: ophcj.nuph.edu.ua/article/download/ophcj.16.905/85180 (accessed on 20 March 2019). [Google Scholar]
- Ozols, A.I.; Pelcher, Y.Ê.; Kalme, Z.A.; Popelis, Y.Y.; Turovskis, I.V.; Duburs, G.Y. Synthesis and chemical properties of 8-aryl-7-acyl-1-6-dimethyl-6-hydroxy-4-cyano-5,6,7,8-tetrahydro-3(2H)-isoquinolinones and isoquinolinethiones. Chem. Heterocycl. Compds. 1996, 32, 52. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Sukach, S.M.; Dyachenko, A.D. Synthesis of partially hydrogenated isoquinoline derivatives by condensation of 2,4-diacetyl-3-aryl(hetaryl)-5-hydroxy-5-methylcyclohexanones with malononitrile and its dimer and a study of their alkylation. Chem. Heterocycl. Compds. 2015, 51, 51. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Karpov, E.N. Synthesis of functionalized alkyl-substituted cyclohexanones, pyridines, and 2,3,5,6,7,8-hexahydroisoquinolines by condensation of aliphatic aldehydes with CH acids. Russ. J. Org. Chem. 2014, 50, 1787. [Google Scholar] [CrossRef]
- Sukach, S.M.; Dyachenko, V.D. Multicomponent synthesis of 3-(alkylsulfanyl)-8-aryl(hetaryl)-7-acetyl-6-hydroxy-1,6-dimethyl-5,6,7,8-tetrahydroisoquinoline-4-carbonitriles. Russ. J. Org. Chem. 2015, 51, 1020. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Sukach, S.M. Synthesis of 7-acetyl-8-aryl(hetaryl)-6-hydroxy-1,6-dimethyl-3-selenoxo- 2,3,5,6,7,8-hexahydroiso-quinoline-4-carbonitriles by the condensation of 2,4-diacetyl-3-aryl(hetaryl)- 5-hydroxy-5-methyl-cyclohexanones with cyanoselenoacetamide. Chem. Heterocycl. Compds. 2010, 46, 1467. [Google Scholar] [CrossRef]
- Gein, V.L.; Nosova, N.V.; Potemkin, K.D.; Aliev, Z.G.; Kriven’ko, A.P. Synthesis and Structure of Diisopropyl 6-Hydroxy-6-methyl-4-oxocyclohexane-1,3-dicarboxylates and Their Reactions with Nucleophilic Reagents. Russ. J. Org. Chem. 2005, 41, 1016. [Google Scholar] [CrossRef]
- Sorokin, V.V.; Grigoryev, A.V.; Ramazanov, A.K.; Krivenko, A.P. Synthesis of 3-R2-4-R1-acetyl- (ethoxycarbonyl)-6-hydroxy-6-methylindazoles. Chem. Heterocycl. Compds. 1999, 35, 671. [Google Scholar] [CrossRef]
- Smirnova, N.O.; Plotnikov, O.P.; Vinogradova, N.A.; Sorokin, V.V.; Kriven’ko, A.P. Synthesis and biological activity of substituted 7-aza-8-aza(oxa)-bicyclo[4.3.0]-6,9-nonadienes. Pharm. Chem. J. 1995, 29, 49. [Google Scholar] [CrossRef]
- Poplevina, N.V.; Kuznetsova, A.A.; Krivenko, A.P. Synthesis and structure of substituted triazoloquinazolines. Chem. Heterocycl. Compds. 2010, 46, 1148. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Sukach, S.M. Synthesis of 1H-pyrazolo[3,4-c]isoquinolin-1-ones by the condensation of cyclohexanone derivatives with 3-amino-1-phenyl-1H-pyrazol-5(4H)-one. Russ. J. Gen. Chem. 2012, 82, 305. [Google Scholar] [CrossRef]
- Anwar, H.F.; Elnagdi, M.H. Recent developments in aminopyrazole chemistry. ARKIVOC 2009, 198. [Google Scholar] [CrossRef]
- Abu Elmaati, T.M.; El-Taweel, F.M. New trends in the chemistry of 5-aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109. [Google Scholar] [CrossRef]
- Etman, H.A.; Sadek, M.G.; Khalil, A.G.M. Synthesis of Some New Heterocycles Derived from 3-Amino-5- hydrazinopyrazole dihydrochloride. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 247. [Google Scholar]
- Dotsenko, V.V.; Ismiev, A.I.; Khrustaleva, A.N.; Frolov, K.A.; Krivokolysko, S.G.; Chigorina, E.A.; Snizhko, A.P.; Gromenko, V.M.; Bushmarinov, I.S.; Askerov, R.K.; et al. Synthesis, structure, and reactions of (4-aryl-3-cyano-6-oxopiperidin-2-ylidene)malononitriles. Chem. Heterocycl. Compds. 2016, 52, 473. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Fused sulfur-containing pyridine systems. 1. Synthesis and structures of tetrahydropyridothienopyridinone and tetrahydropyridothiopyranopyridinone derivatives. Russ. Chem. Bull. Int. Ed. 2003, 52, 969. [Google Scholar] [CrossRef]
- Tverdokhleb, N.M.; Khoroshilov, G.E.; Dotsenko, V.V. Cascade synthesis of pyrido[3,2-a]indolizines by reaction of Kröhnke–Mukaiyama salts with malononitrile dimer. Tetrahedron Lett. 2014, 55, 6593. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Chigorina, E.A.; Krivokolysko, S.G. Synthesis of derivatives of a novel heterocyclic system 7-thia-1,4,6,8-tetraazabenzo[de]anthracene. Chem. Heterocycl. Compds. 2017, 53, 626. [Google Scholar] [CrossRef]
- Carboni, R.A.; Coffman, D.D.; Howard, E.G. Cyanocarbon Chemistry. XI.1 Malononitrile Dimer. J. Am. Chem. Soc. 1958, 80, 2838. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Semenova, A.M.; Chigorina, E.A.; Aksenov, N.A.; Aksenova, I.V. The Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazol-4-carbonitrile with beta-Cycloketols. Proceedings 2019, 9, 25. https://doi.org/10.3390/ecsoc-22-05679
Dotsenko VV, Semenova AM, Chigorina EA, Aksenov NA, Aksenova IV. The Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazol-4-carbonitrile with beta-Cycloketols. Proceedings. 2019; 9(1):25. https://doi.org/10.3390/ecsoc-22-05679
Chicago/Turabian StyleDotsenko, Victor V., Aminat M. Semenova, Elena A. Chigorina, Nikolai A. Aksenov, and Inna V. Aksenova. 2019. "The Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazol-4-carbonitrile with beta-Cycloketols" Proceedings 9, no. 1: 25. https://doi.org/10.3390/ecsoc-22-05679
APA StyleDotsenko, V. V., Semenova, A. M., Chigorina, E. A., Aksenov, N. A., & Aksenova, I. V. (2019). The Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazol-4-carbonitrile with beta-Cycloketols. Proceedings, 9(1), 25. https://doi.org/10.3390/ecsoc-22-05679




