The First Synthesis of [1,2]oxaphosphinino[6,5-c]pyrazoles by Thiophosphorylation of 6-Aminopyrano[2,3-c]pyrazole-5-Carbonitriles †
Abstract
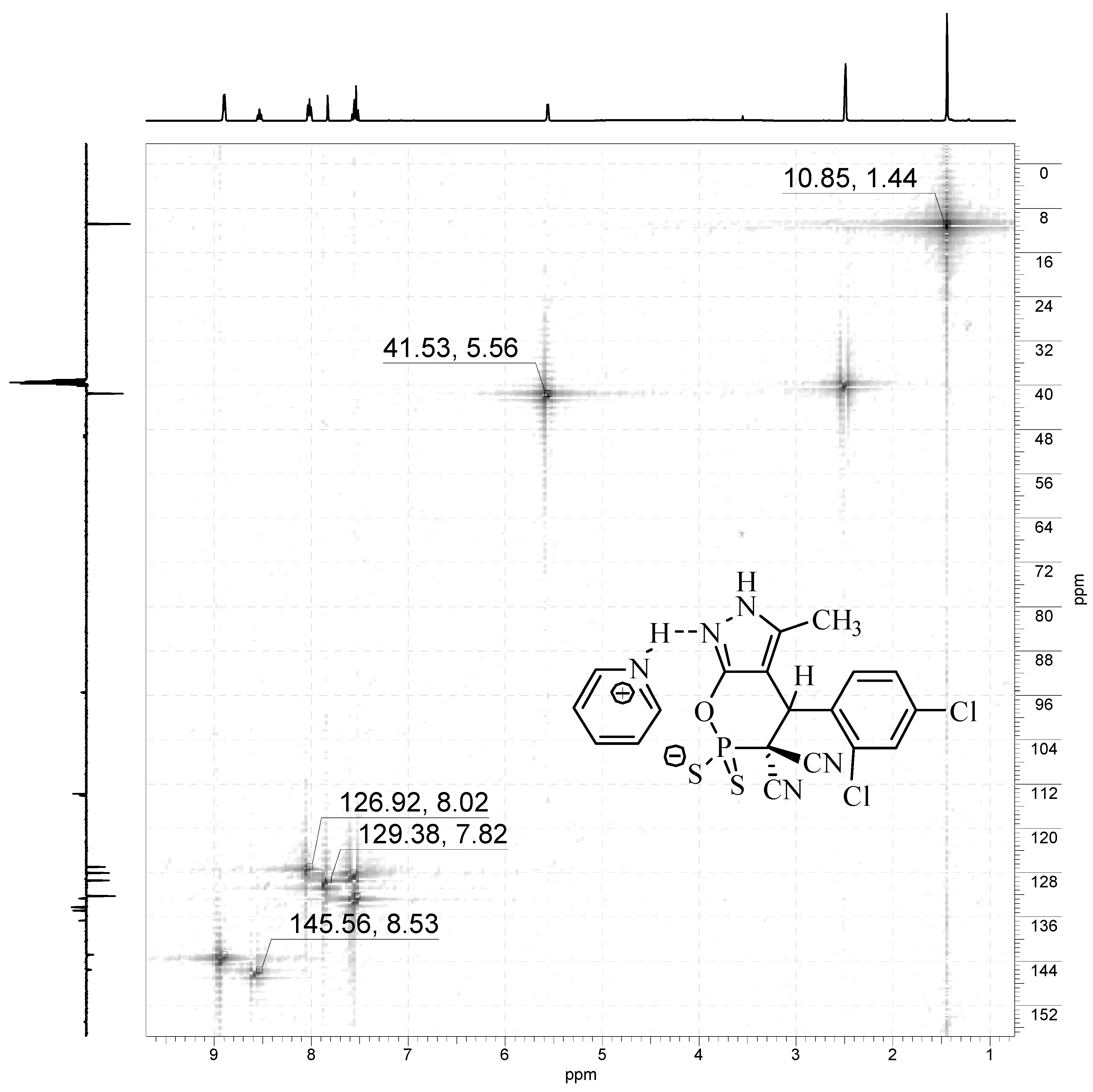
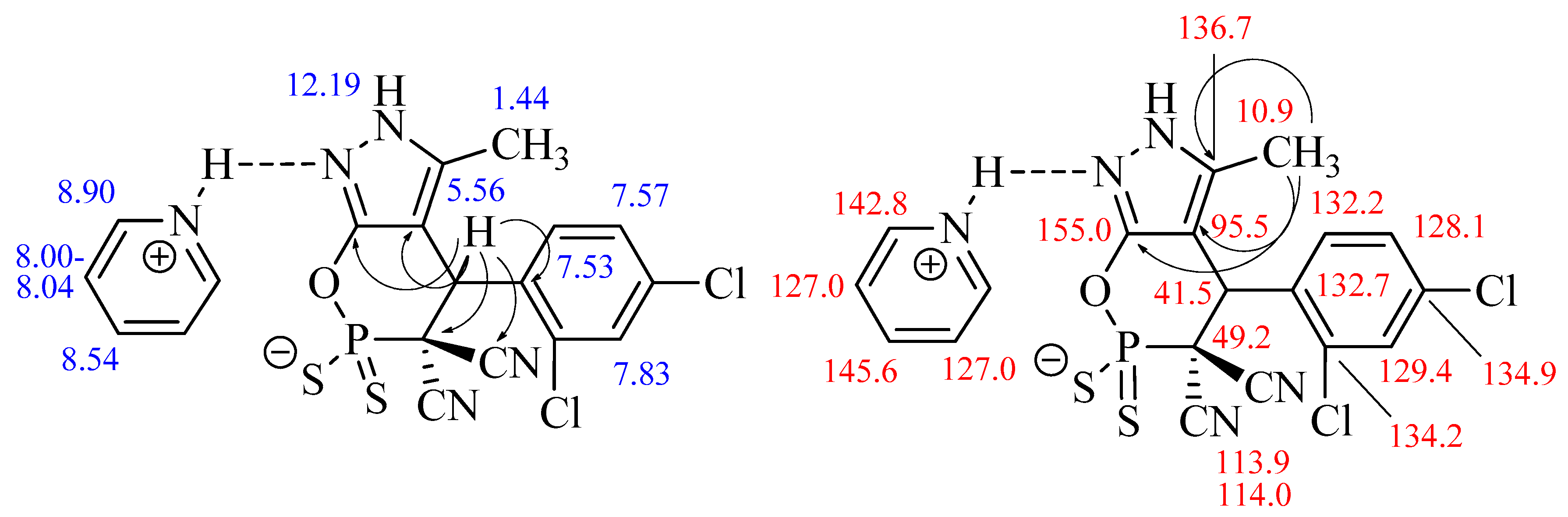

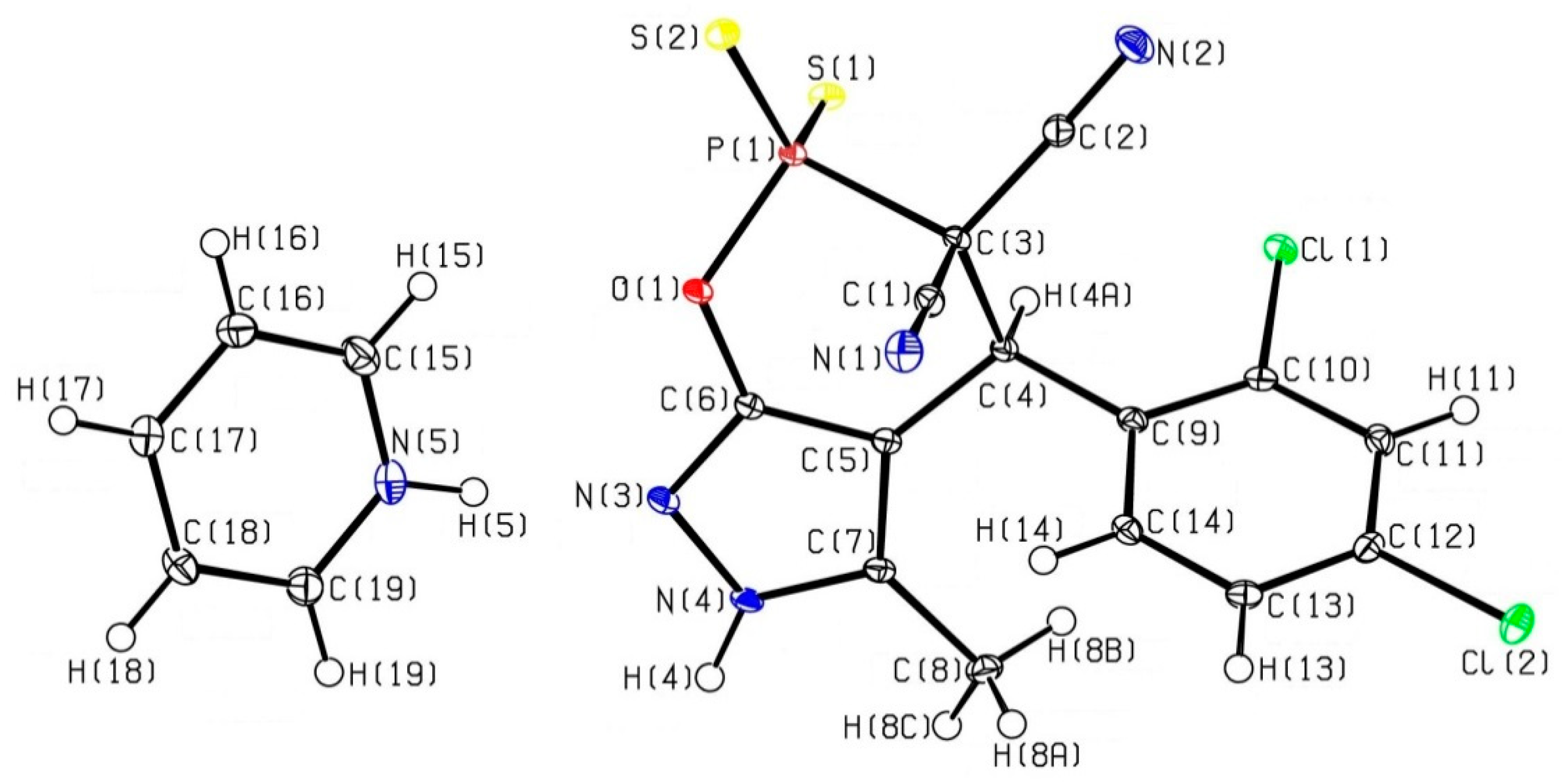
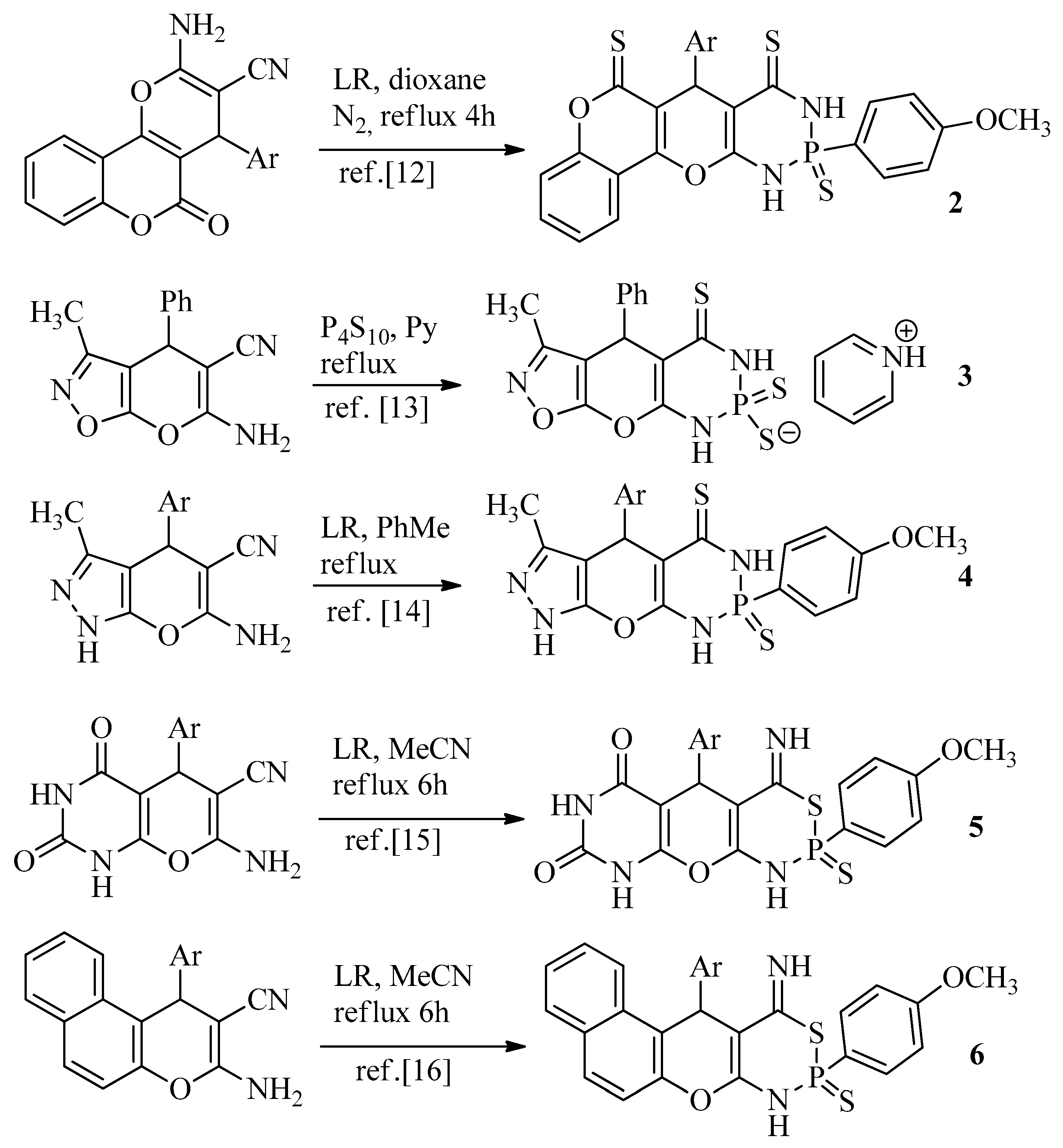
Experimental
References
- Myrboh, B.; Mecadon, H.; Rohman, M.R.; Rajbangshi, M.; Kharkongor, I.; Laloo, B.M.; Kharbangar, I.; Kshiar, B. Synthetic Developments in Functionalized Pyrano[2,3-c]pyrazoles. A Review. Org. Prep. Proced. Int. 2013, 45, 253. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Goncharenko, M.P.; Litvinov, V.P. Reactions of carbonyl compounds with α,β-unsaturated nitriles as a convenient pathway to carbo- and heterocycles. Russ. Chem. Rev. 1998, 67, 393. [Google Scholar] [CrossRef]
- Shestopalov, A.M.; Emeliyanova, Y.M. Selected Methods for Synthesis and Modification of Heterocycles; Kartsev, V.G., Ed.; IBS Press: Moscow, Russia, 2003; Volume 2, p. 363. (In Russian) [Google Scholar]
- Litvinov, Yu.M.; Shestopalov, A.M. Synthesis, Structure, Chemical Reactivity, and Practical Significance of 2-Amino-4H-pyrans. Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Oxford, UK, 2011; Volume 103, p. 175. [Google Scholar] [CrossRef]
- Kozachenko, A.P.; Shablykin, O.V.; Gakh, A.A.; Rusanov, E.B.; Brovarets, V.S. Synthesis of new heterocyclic system of 4,5,7,8-tetrahydroimidazo[1,2-c][1,3]thiazolo [4,5-e][1,3,2]diazaphosphinine starting from 2-aroylamino-3,3-dichloroacrylonitrile. Heteroatom Chem. 2010, 21, 492. [Google Scholar] [CrossRef]
- Khalladi, K.; Touil, S. Synthesis of novel fused thienodiazaphosphorine derivatives from 2-amino-3-cyanothiophenes and Lawesson's reagent. J. Sulfur Chem. 2012, 33, 27. [Google Scholar] [CrossRef]
- Allouche, F.; Chabchoub, F.; Salem, M.; Kirsch, G. Synthesis of New Pyrazolopyrimidinedithiones and Pyrazolopyrimidinephosphines from Aminocyanopyrazoles. Synthetic Commun. 2011, 41, 1500. [Google Scholar] [CrossRef]
- Nilov, D.B.; Kadushkin, A.V.; Solov’eva, N.P.; Sheinker, Y.N.; Granik, V.G. Synthesis and Study of the Properties of 7,8-Polymethyleneimidazo[4,5-d]-1,3,2-diazaphosphorin-2-thiones. Chem. Heterocycl. Compds. 2004, 40, 106. [Google Scholar] [CrossRef]
- Nilov, D.B.; Kadushkin, A.V.; Solov’eva, N.P.; Sedov, A.L.; Granik, V.G. Interaction of P2S5–pyridine with enamines. Synthesis and reactions of l,6-trimethylene-5-cyano-2-mercapto-l,3,2-diaza- phosphorine-2-thione. Mendeleev Commun. 1996, 6, 191. [Google Scholar] [CrossRef]
- Nilov, D.B.; Kadushkin, A.V.; Granik, V.G. Chemical Properties of 1,6-Polymethylene-5-cyano-1,3,2 5 -Diazaphosphinane-2,4-Dithiones Synthesized Via Reactions of Enamines with P2S5/Pyridine System Pharm. Chem. J. 2004, 38, 451. [Google Scholar] [CrossRef]
- Elgazwy, A.S.S.H.; Soliman, D.H. Design, Synthesis and Evaluation of 1,3,2-Diazaphosphorin[4,5- b]quinoxaline-5,10-di-N-oxide derivatives as novel VEGFR-2 and SRC kinase inhibitors in the treatment of prostate cancer. Open Conf. Proc. J. 2013, 4, 77. [Google Scholar] [CrossRef][Green Version]
- Gardelly, M.; Trimech, B.; Belkacem, M.A.; Harbach, M.; Abdelwahed, S.; Mosbah, A.; Bouajila, J.; Jannet, H.B. Synthesis of novel diazaphosphinanes coumarin derivatives with promoted cytotoxic and anti-tyrosinase activities. Bioorg. Med. Chem. Lett. 2016, 26, 2450. [Google Scholar] [CrossRef]
- Ali, T.E.; Assiri, M.A.; Abdel-Kariem, S.M.; Yahia, I.S. Facile synthesis of novel 6-methyl-5-phenyl-2-sulfido-1,2,3,5-tetrahydro-4H[1,2]oxazolo[4′,5′:5,6]pyrano[2,3-d][1,3,2]diazaphosphi-nines. J. Sulfur Chem. 2018, 39, 482. [Google Scholar] [CrossRef]
- Mohamed, N.R.; Khaireldin, N.Y.; Fahmy, A.F.; El-Sayed, A.A. The Utility of Carbon Disulphide and Lawesson's Reagent for Synthesis of Different Fused Heterocycles for Antimicrobial Evaluation. J. Heterocycl. Chem. 2013, 50, 1264. [Google Scholar] [CrossRef]
- Younes, S.H.H.; Mohamed, S.K.; Albayati, M.R. Studies on organophosphorus compounds. Part 1: Synthesis and in vitro antimicrobial activity of some new pyrimido[5′,4′:5,6]pyrano[2,3-d][1,3,2]- thiazaphosphinine compounds. Arch. Pharm. 2013, 346, 727. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.H.; Mohamed, S.K.; Abdelhamid, A.A.; Ghattas, A.-B.A.G. Studies on Organophosphorus Compounds Part II: Synthesis and biological activities of some new benzochromeno[2,3-d][1,3,2]thiazaphosphinine derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 81. Available online: http://globalresearchonline.net/journalcontents/v23-2/15.pdf (accessed on 20 March 2019).
- Dotsenko, V.V.; Krivokolysko, S.G. Synthesis of pyrido[3',2':4,5]thieno[3,2-d][1,3,2]diazaphosphorine derivatives. Chem. Heterocycl. Compds. 2012, 48, 1863. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Dushenko, V.A.; Aksenov, N.A.; Aksenova, I.V.; Netreba, E.E. The First Synthesis of [1,2]oxaphosphinino[6,5-c]pyrazoles by Thiophosphorylation of 6-Aminopyrano[2,3-c]pyrazole-5-Carbonitriles. Proceedings 2019, 9, 26. https://doi.org/10.3390/ecsoc-22-05680
Dotsenko VV, Dushenko VA, Aksenov NA, Aksenova IV, Netreba EE. The First Synthesis of [1,2]oxaphosphinino[6,5-c]pyrazoles by Thiophosphorylation of 6-Aminopyrano[2,3-c]pyrazole-5-Carbonitriles. Proceedings. 2019; 9(1):26. https://doi.org/10.3390/ecsoc-22-05680
Chicago/Turabian StyleDotsenko, Victor V., Vladimir A. Dushenko, Nikolai A. Aksenov, Inna V. Aksenova, and Evgeniy E. Netreba. 2019. "The First Synthesis of [1,2]oxaphosphinino[6,5-c]pyrazoles by Thiophosphorylation of 6-Aminopyrano[2,3-c]pyrazole-5-Carbonitriles" Proceedings 9, no. 1: 26. https://doi.org/10.3390/ecsoc-22-05680
APA StyleDotsenko, V. V., Dushenko, V. A., Aksenov, N. A., Aksenova, I. V., & Netreba, E. E. (2019). The First Synthesis of [1,2]oxaphosphinino[6,5-c]pyrazoles by Thiophosphorylation of 6-Aminopyrano[2,3-c]pyrazole-5-Carbonitriles. Proceedings, 9(1), 26. https://doi.org/10.3390/ecsoc-22-05680




