Recovery of Bioactive Compounds from Exhausted Olive Pomace †
Abstract
:1. Introduction
2. Methods
2.1. Chemical and Standards
2.2. Raw Material
2.3. Conditions Applied in the Extraction Methods
2.4. Total Phenolic Content
2.5. Phenolic Profile by HPLC
3. Results and Discussion
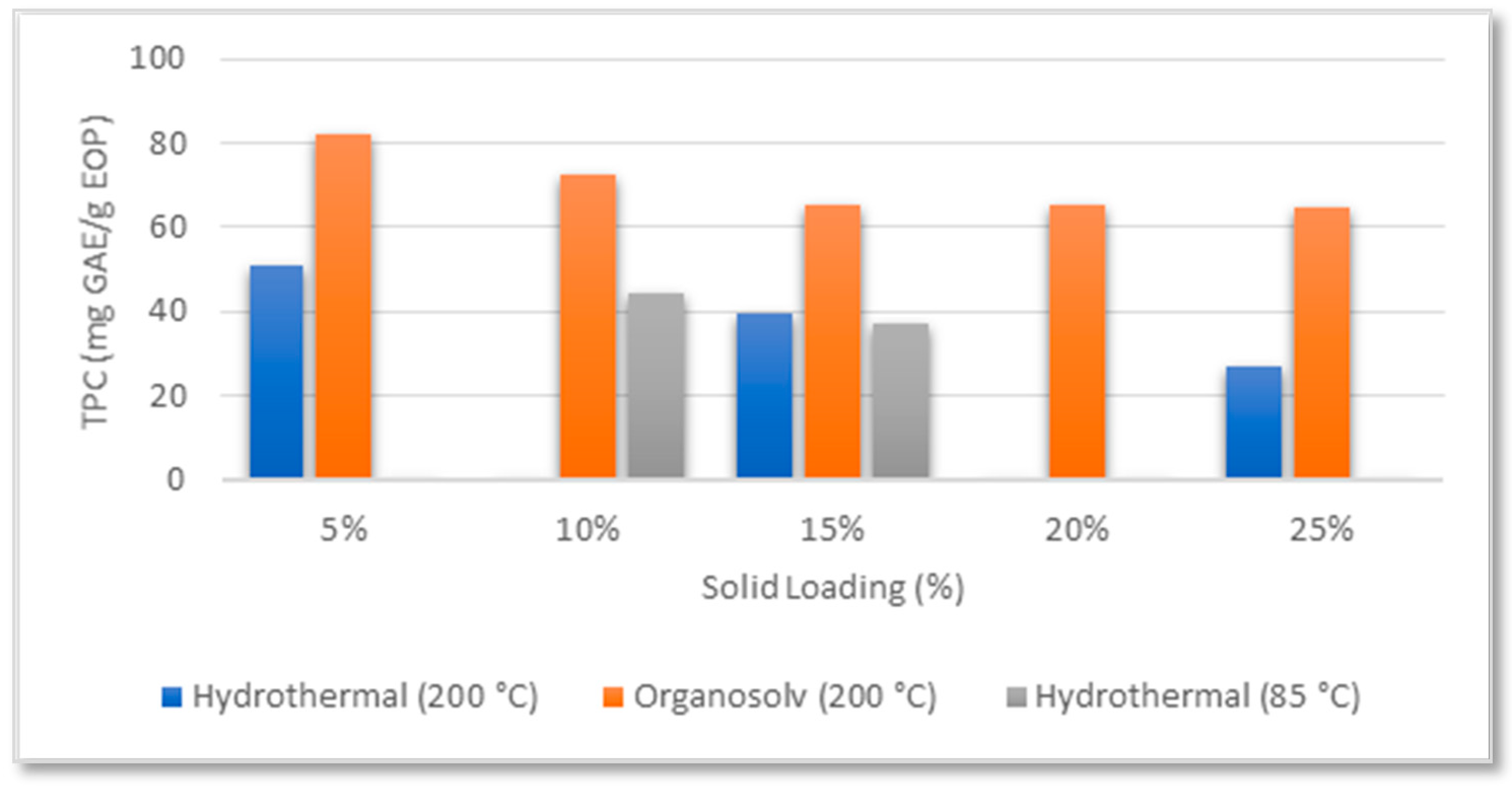
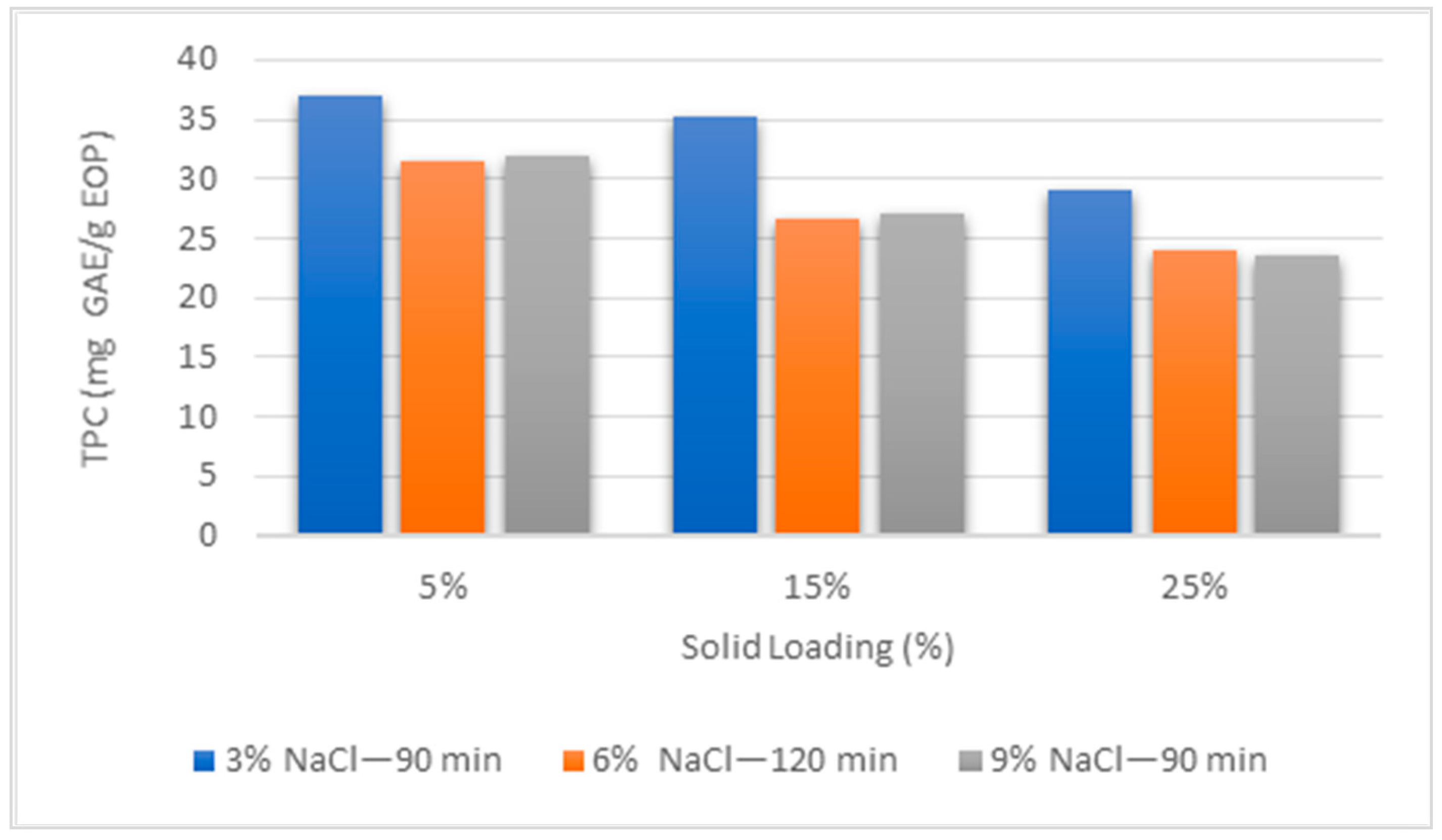
3.1. Evaluation of the Extraction Conditions
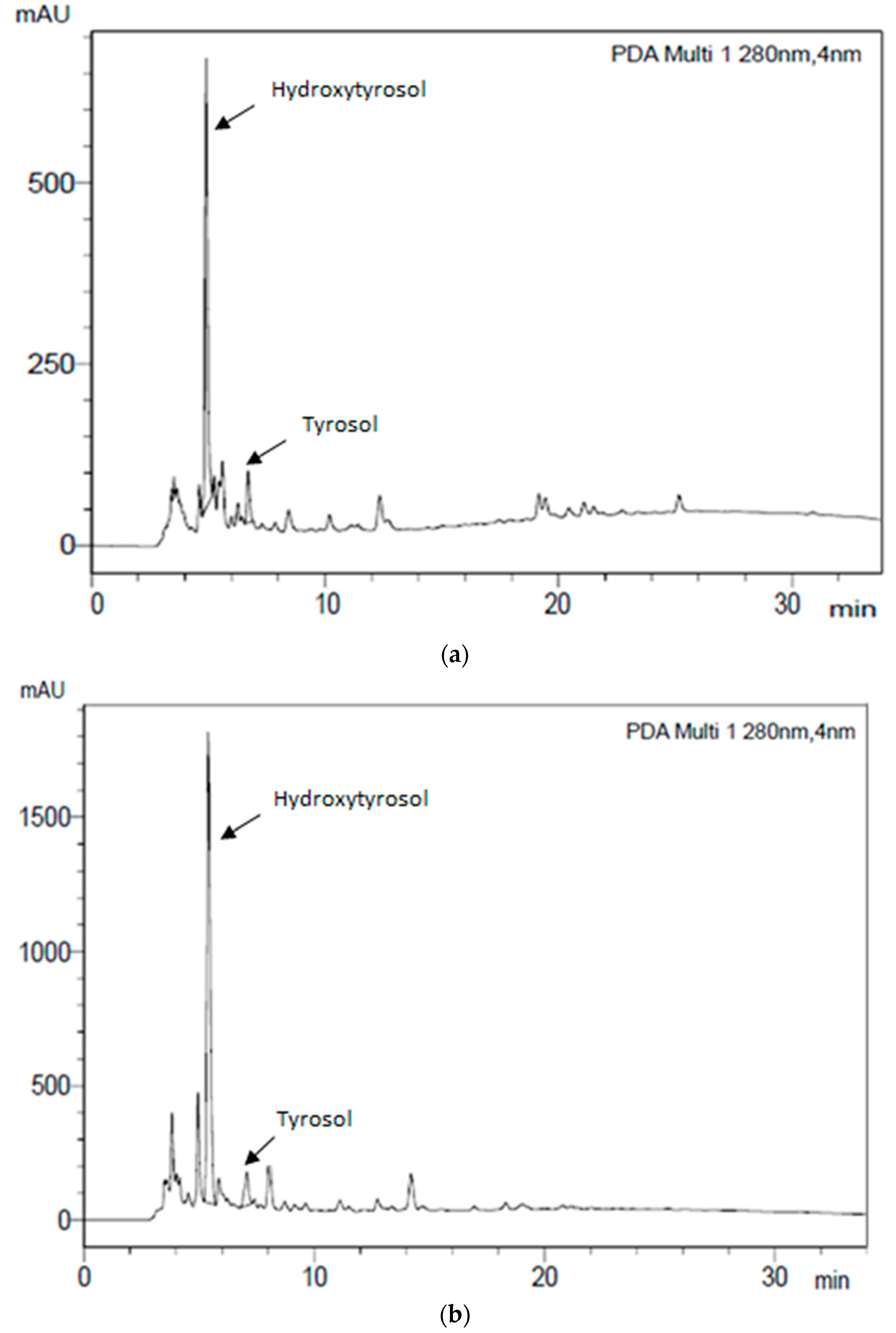
3.2. Phenolic Profiles
4. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz:, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuel Bioprod. Biorefin. 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual biomass potential in olive tree cultivation and olive oil industry in Spain: Valorization proposal in a biorefinery context. Span J. Agric. Res. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gómez-Cruz, I.; Romero, I.; Gullón, B.; Ruiz, E.; Brnčićc, M.; Castro, E. Ultrasound-assisted extraction as a first step in a biorefinery strategy for valorisation of extracted olive pomace. Energies 2019, 12, 2679. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, 40–47. [Google Scholar] [CrossRef]
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rietjens, S.J.; Bast, A.; Haenen, G.R.M.M. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Optimization of oleuropein and luteolin-7-O-glucoside extraction from olive leaves by ultrasound-assisted technology. Energies 2019, 12, 2486. [Google Scholar] [CrossRef]
- Díaz, M.J.; Huijgen, W.J.J.; Van, R.R.; Laan, D.; Reith, J.H.; Cara, C.; Castro, E. Organosolv pretreatment of olive tree biomass for fermentable sugars. Holzforschung 2011, 65, 177–183. [Google Scholar] [CrossRef]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2009, 114, 806–812. [Google Scholar] [CrossRef]
- Pearson, C.H.; Cornish, K.; Rath, D.J. Extraction of natural rubber and resin from guayule using an accelerated solvent extractor. Ind. Crops Prod. 2013, 43, 506–510. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Ahmed, I.; Ahmad, M. Olive oil and its principal bioactive compound : Hydroxytyrosol—A review of the recent literature. Trends Food Sci Technol 2018, 77, 77–90. [Google Scholar] [CrossRef]
| Extraction Method | Solvent | Solvent (%, v/v) | Temperature (°C) | Time (min) | Solids (%) |
|---|---|---|---|---|---|
| Hydrothermal extraction | Water | 100 | 85 | 90 | 10, 15 |
| 100 | 200 | 0 b | 5, 15, 25 | ||
| Organosolv extraction | Ethanol a | 50 | 200 | 0 b | 5, 10, 15, 20, 25 |
| Accelerated extraction | Water | 100 | 55 | 30 | 2 |
| 100 | 190 | 10 | 2 | ||
| Extraction with aqueous salt solution | Sodium chloride a | 3, 9 | 55 | 90 | 5, 15, 25 |
| 6 | 55 | 120 | 5, 15, 25 |
| Cycle a | Temperature (°C) | Time (min) | Solid Loading (%) | TPC (mg Gallic Acid Equivalents/g EOP) |
|---|---|---|---|---|
| 1 | 55 | 30 | 25 | 35.6 |
| 2 | 55 | 30 | 25 | 6.3 |
| 1 | 190 | 10 | 25 | 40.6 |
| 2 | 190 | 10 | 25 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cruz, I.; Cara, C.; Contreras, M.d.M.; Romero, I. Recovery of Bioactive Compounds from Exhausted Olive Pomace. Proceedings 2021, 79, 9. https://doi.org/10.3390/IECBM2020-08582
Gómez-Cruz I, Cara C, Contreras MdM, Romero I. Recovery of Bioactive Compounds from Exhausted Olive Pomace. Proceedings. 2021; 79(1):9. https://doi.org/10.3390/IECBM2020-08582
Chicago/Turabian StyleGómez-Cruz, Irene, Cristóbal Cara, María del Mar Contreras, and Inmaculada Romero. 2021. "Recovery of Bioactive Compounds from Exhausted Olive Pomace" Proceedings 79, no. 1: 9. https://doi.org/10.3390/IECBM2020-08582
APA StyleGómez-Cruz, I., Cara, C., Contreras, M. d. M., & Romero, I. (2021). Recovery of Bioactive Compounds from Exhausted Olive Pomace. Proceedings, 79(1), 9. https://doi.org/10.3390/IECBM2020-08582







