Analytical Investigation of Cyclodextrin Complexation Using the Co-Grinding Technique in the Case of Terbinafine Hydrochloride †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Preparation of Co-Ground Mixtures
2.2.2. Differential Scanning Calorimetry (DSC)
2.2.3. X-ray Powder Diffractometry (XRPD)
2.2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.5. Raman Spectroscopy
2.2.6. Dissolution Rate Studies
3. Results and Discussion
3.1. Differential Scanning Calorimetry
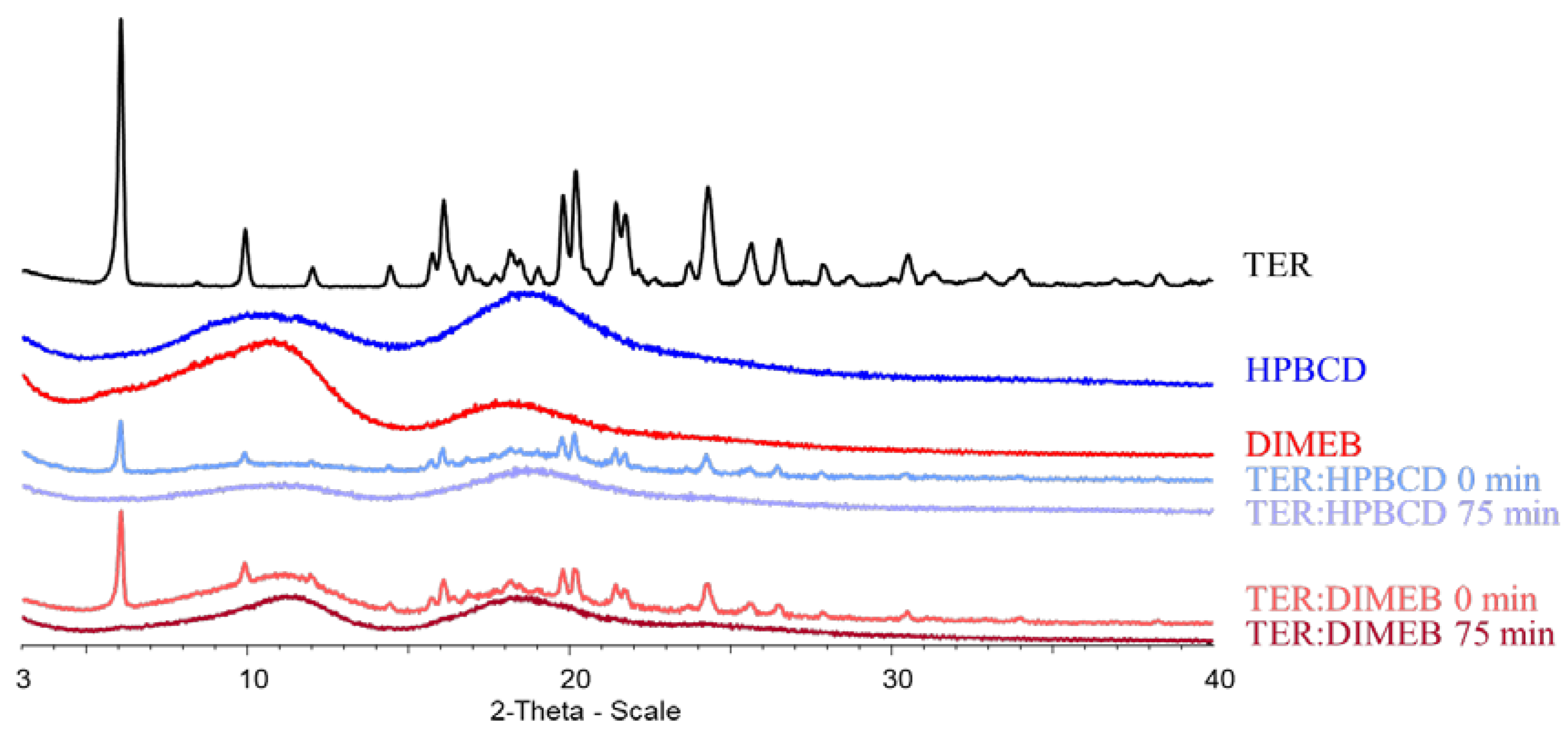
3.2. X-ray Powder Diffractometry
3.3. Hot-Humidity X-ray Powder Diffractometry (HOT-XRPD)
3.4. Fourier-Transform Infrared Spectroscopy
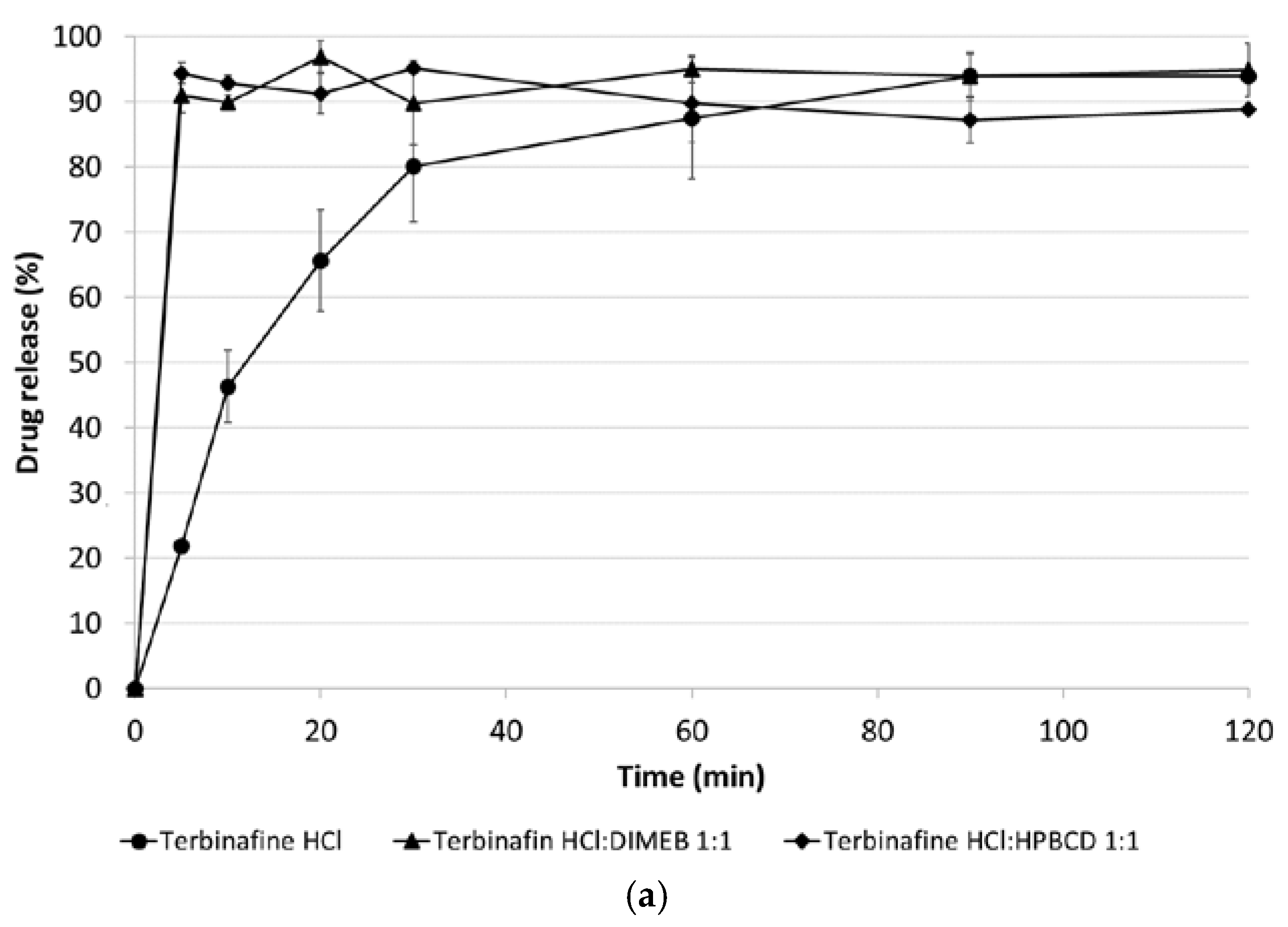
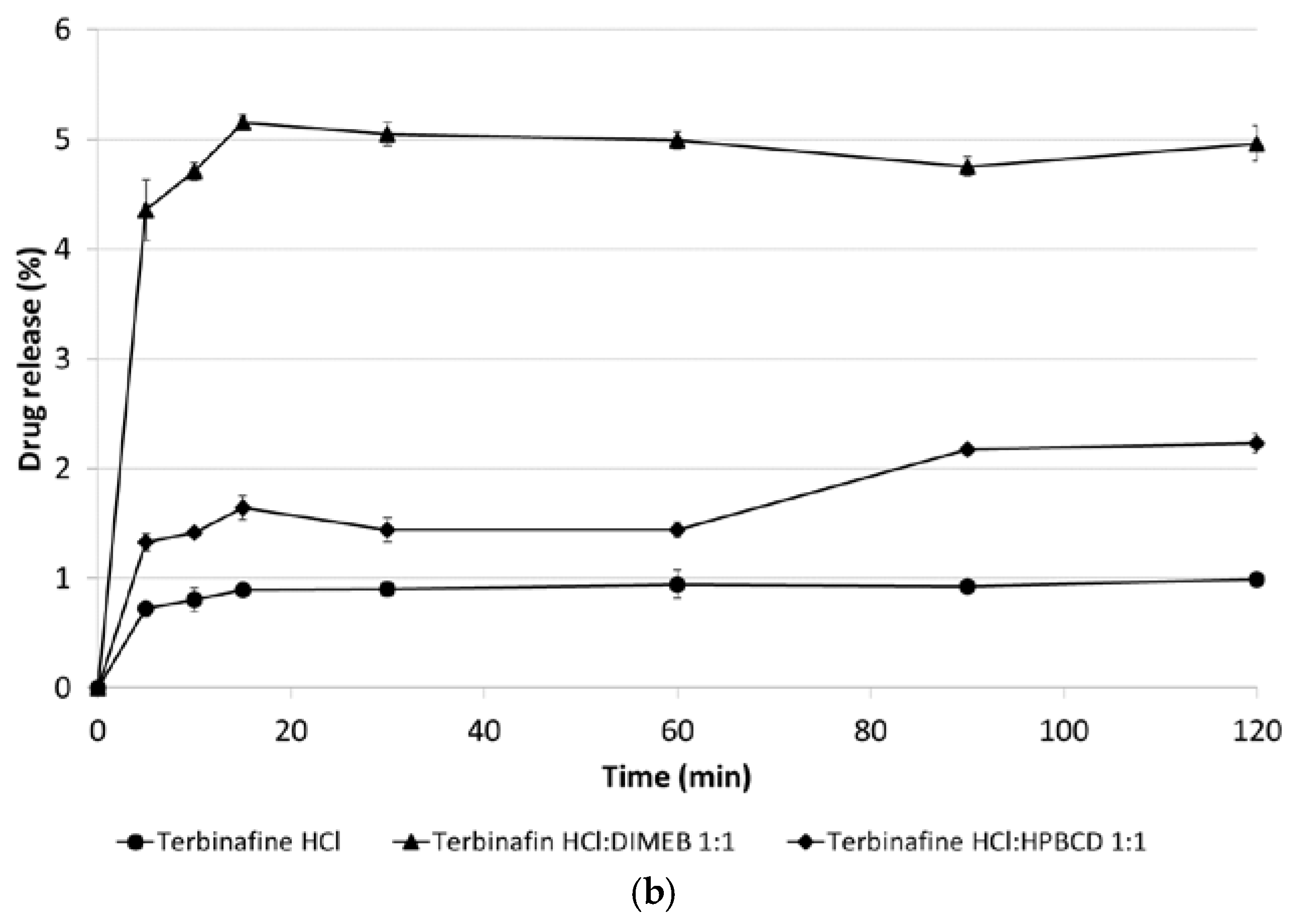
3.5. Dissolution Studies
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCS | Biopharmaceutics Classification System |
| CD | cyclodextrin |
| TER | terbinafine hydrochloride |
| HPBCD | hydroxypropyl-β-cyclodextrin |
| DIMEB | heptakis(2,6-di-O-methyl)-β-cyclodextrin |
| DSC | differential scanning calorimetry |
| XRPD | X-ray powder diffractometry |
| HOT-XRPD | hot-humidity stage X-ray powder diffractometry |
| FT-IR | Fourier-transform infrared spectroscopy |
References
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 45, 167–180. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. on Drug Delivery 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondoros, B.A.; Laczkovich, O.; Berkesi, O.; Aigner, Z. Analytical Investigation of Cyclodextrin Complexation Using the Co-Grinding Technique in the Case of Terbinafine Hydrochloride. Proceedings 2021, 78, 19. https://doi.org/10.3390/IECP2020-08714
Kondoros BA, Laczkovich O, Berkesi O, Aigner Z. Analytical Investigation of Cyclodextrin Complexation Using the Co-Grinding Technique in the Case of Terbinafine Hydrochloride. Proceedings. 2021; 78(1):19. https://doi.org/10.3390/IECP2020-08714
Chicago/Turabian StyleKondoros, Balázs Attila, Orsolya Laczkovich, Ottó Berkesi, and Zoltán Aigner. 2021. "Analytical Investigation of Cyclodextrin Complexation Using the Co-Grinding Technique in the Case of Terbinafine Hydrochloride" Proceedings 78, no. 1: 19. https://doi.org/10.3390/IECP2020-08714
APA StyleKondoros, B. A., Laczkovich, O., Berkesi, O., & Aigner, Z. (2021). Analytical Investigation of Cyclodextrin Complexation Using the Co-Grinding Technique in the Case of Terbinafine Hydrochloride. Proceedings, 78(1), 19. https://doi.org/10.3390/IECP2020-08714



