Ultra-Short Cyclo-Peptides as Bio-Inspired Therapeutics: Proline-Based 2,5-Diketopiperazines (DKP) †
Abstract
:1. Introduction
2. Cyclo-Peptides: General Considerations
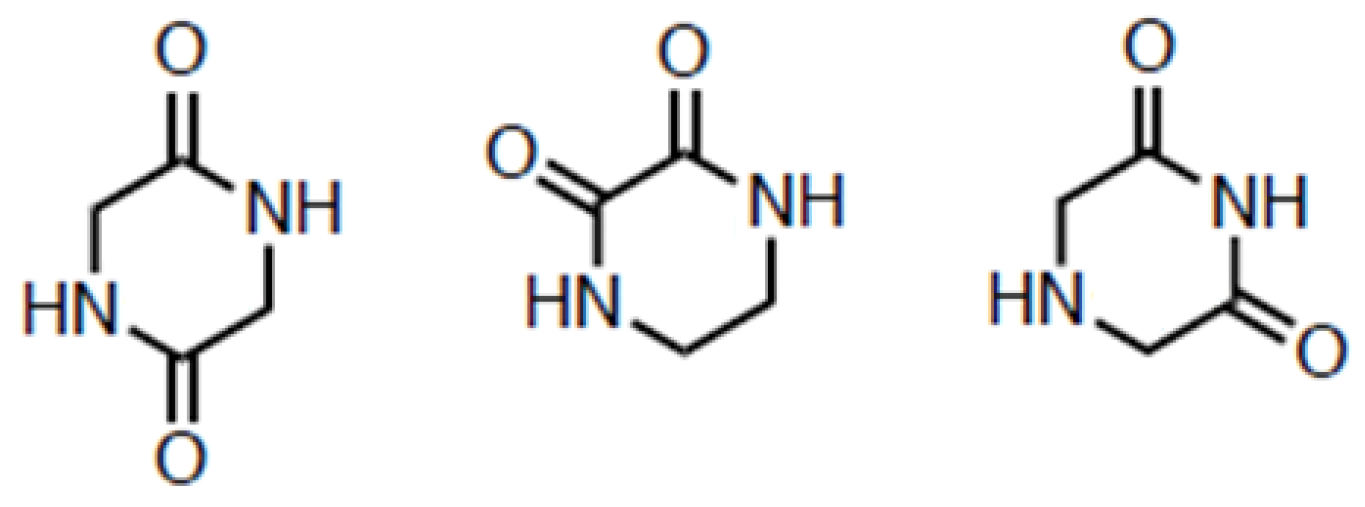
3. Diketopiperazines
3.1. Historical Background and Occurence: Origin of Life
3.2. Properties and Possibilities
3.3. DKP-Based Drugs
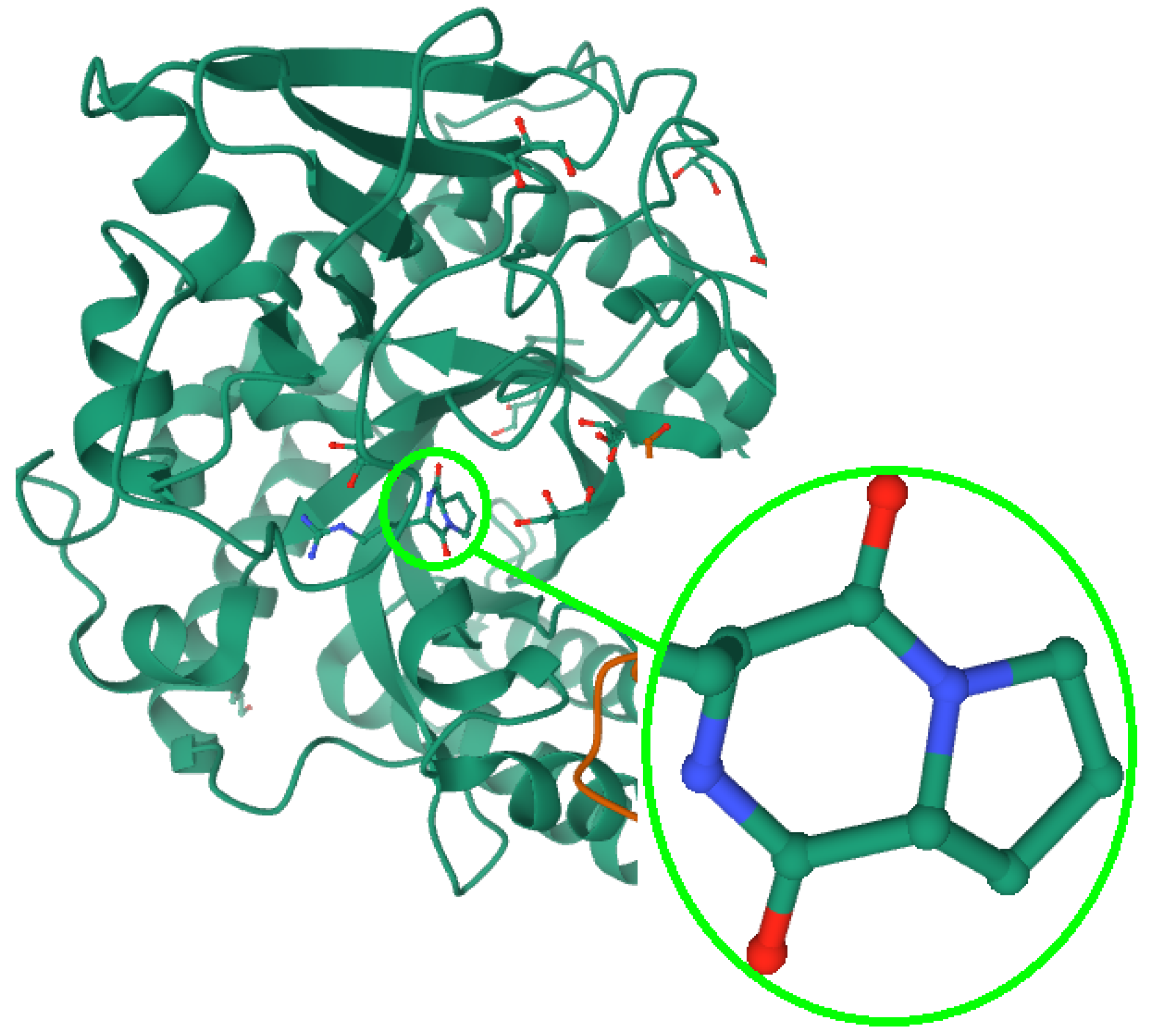
3.4. Cyclo-Dipeptides Containing Proline: Towards Effective Therapies
3.5. Databases Survey
4. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.; Kim, D.W.; Park, H.S. Cyclic peptides: Promising scaffolds for biopharmaceuticals. Genes 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Feni, L.; Jutten, L.; Parente, S.; Piarulli, U.; Neundorf, I.; Dia, D. Cell-penetrating peptides containing 2,5-DKP scaffolds as shuttles for anti-cancer drugs: Conformational studies and biological activities. Chem. Commun. 2020, 56, 5685–5688. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Giessen, T.W.; Marahiel, M.A. Rational and combinatorial tailoring of bioactive cyclic dipeptides. Front. Microbiol. 2015, 6, 785. [Google Scholar] [CrossRef]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef]
- Sano, S.; Nakao, M. Chemistry of 2,5-diketopiperazine and its bis-lactim ether: A brief review. Heterocycles 2015, 91, 1349–1375. [Google Scholar] [CrossRef]
- Ying, J.; Lin, R.; Xu, P.; Wu, Y.; Zhao, Y. Prebiotic formation of cyclic dipeptides under potentially early Earth conditions Sci. Rep. 2018, 8, 936. [Google Scholar]
- Gondry, M.; Sauguet, L.; Belin, P.; Thai, R.; Amouroux, R.; Tellier, C.; Tuphile, K.; Jacquet, M.; Braud, S.; Courcon, M.; et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 2009, 5, 414–420. [Google Scholar] [CrossRef]
- García-Estrada, C.; Ullán, R.; Albillos, S.; Fernández-Bodega, M.; Durek, P.; Vondöhren, H.; Martín, J. A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem. Biol. 2011, 18, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Borgman, P.; Lopez, R.D.; Lane, A.L. The expanding spectrum of DKP natural product biosynthetic pathways containing cyclodipeptide synthases. Org. Biomol. Chem. 2019, 17, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Moutiez, M.; Belin, P.; Gondry, M. Aminoacyl-tRNA-Utilizing Enzymes in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5578–5618. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Schoppet, M.; Hansen, M.H.; Cryle, M.J. Diversity of nature's assembly lines – recent discoveries in non-ribosomal peptide synthesis. Mol. BioSyst. 2016, 13, 9–22. [Google Scholar] [CrossRef]
- Elkahoui, S.; Abdel Rahim, H.; Tabbene, O.; Shaaban, M.; Limam, F.; Laatsch, H. Cyclo-(His,Leu): A new microbial diketopiperazine from a terrestrial Bacillus subtilis strain B38. Nat. Prod. Res. 2013, 27, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Ram Chhabra, S.; de Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D.; et al. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol. Microbiol. 1999, 33, 1254–1266. [Google Scholar] [CrossRef]
- Ström, K.; Sjögren, M.; Broberg, A.; Schnürer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Li, J.; Li, X.; Zu, X.; Zhao, Z.; Quan, C.; Gao, W.; Feng, S. Non-lipopeptide fungi-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2019, 41, 651–673. [Google Scholar] [CrossRef]
- Lin, A.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. A new diketopiperazine alkaloid isolated from an algicolous Aspergillus flavus strain. Pharmazie 2008, 63, 323–325. [Google Scholar]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternate. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Wells, P.J. A diketopiperazine derived from trichloroleucine from the sponge Dysidea herbacea. Tetrahedron Lett. 1978, 49, 4945–4948. [Google Scholar] [CrossRef]
- Prasad, C. Cyclo(His-Pro): Its distribution, origin and function in the human. Neurosci. Biobehav. Rev. 1988, 12, 19–22. [Google Scholar] [CrossRef]
- Huang, R.M.; Yi, X.X.; Zhou, Y.; Su, X.; Pengs, Y.; Gao, C.H. An Update on 2,5-Diketopiperazines from Marine Organisms. Mar. Drugs 2014, 12, 6213–6235. [Google Scholar] [CrossRef] [PubMed]
- Harizani, M.; Katsini, E.; Georgantea, P.; Roussis, V.; Ioannou, E. New chlorinated 2,5DKPs from marine-derived bacteria isolated from sediments of the eastern Mediterranean sea. Molecules 2020, 25, 1509. [Google Scholar] [CrossRef] [PubMed]
- Ginz, M.; Engelhardt, U.H. Identification of proline-based diketopiperazines in roasted coffee. J. Agric. Food Chem. 2000, 48, 3528–3532. [Google Scholar] [CrossRef]
- Chen, M.Z.; Dewis, M.L.; Kraut, K.; Merritt, D.; Reiber, L.; Trinnaman, L.; Da Costa, N.C. 2,5-Diketopiperazines (cyclic dipeptides) in beef: Identification, synthesis, and sensory evaluation. J. Food Sci. 2009, 74, C100–C105. [Google Scholar] [CrossRef]
- Gautschi, M.; Schmid, J.P.; Peppard, T.L.; Ryan, T.P.; Tuorto, R.M.; Yang, X. Chemical characterization of diketopiperazines in beer. J. Agric. Food Chem. 1997, 45, 3183–3189. [Google Scholar] [CrossRef]
- Otsuka, Y.; Arita, H.; Sakaji, M.; Yamamoto, K.; Kashiwagi, T.; Shimamura, T.; Ukeda, H. Investigation of the formation mechanism of proline-containing cyclic dipeptide from the linear peptide. Biosci. Biotechnol. Biochem. 2019, 12, 2355–2363. [Google Scholar] [CrossRef]
- Bojarska, J.; Remko, L.; Maniukiewicz, W.; Sieron, M. An orthorhombic polymorph of a cyclization product of perindopril. Acta Crystallogr. C 2013, 69, 630–633. [Google Scholar] [CrossRef]
- Bojarska, J.; Maniukiewicz, W.; Główka, M.L.; Siero’n, L.; Remko, M. Crystal structure of perindopril cyclization product. J. Chil. Chem. Soc. 2013, 58, 1530–1532. [Google Scholar] [CrossRef]
- Remko, M.; Bojarska, J.; Jezko, L.; Olczak, A.; Maniukiewicz, W. Molecular structure of antihypertensive drug perindopril, its active metabolite perindoprilat and impurity F. J. Mol. Struct. 2013, 1036, 292–297. [Google Scholar] [CrossRef]
- Yue, L.; Fangfang, L.; Yanyan, Z.; Li, X.; Zhou, Z.; Liu, C.; Zhang, W.; Tang, M. DFT study onreaction mechanisms of cyclic dipeptide generation. Struct. Chem. 2016, 27, 1165–1173. [Google Scholar]
- Corey, R. Diketopiperazine. J. Am. Chem. Soc. 1938, 60, 1598. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. Mini Reviews in Medicinal Chemistry. BenthamScience 2021, 21. [Google Scholar]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Ma, H.; Zhu, W. Developments around the bioactive diketopiperazines: A patent review. Expert Opin. Ther. Patnets 2013, 23, 1415–1433. [Google Scholar] [CrossRef]
- Mishra, A.K.; Choi, J.; Choi, S.J.; Baek, K.H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef]
- Sun, S.J.; Liu, Y.C.; Weng, C.H.; Sun, S.W.; Li, F.; Li, H.; Zhu, H. Cyclic dipeptides mediating quorum sensing and their bilogical effects in Hypsizygus Marmoreus. Biomolecules 2020, 10, 298. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Zhao, X. Neuroprotective e_ects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef]
- Fischer, P.M. Diketopiperazines in peptide and combinatorial chemistry. J. Peptide Sci. 2003, 9, 9–35. [Google Scholar] [CrossRef]
- Dubois, P.; Correia, I.; Le Chevalier, F.; Dubois, S.; Jacques, I.; Canu, N.; Moutiez, M.; Thai, R.; Gondry, M.; Lequin, O.; et al. Reprogramming Escherichia coli for the production of prenylated indole diketopiperazine alkaloids. Sci. Rep. 2019, 9, 9208–9220. [Google Scholar] [CrossRef] [PubMed]
- Ressurreição, A.S.M.; Delatouche, R.; Gennari, C.; Piarulli, U. Bifunctional 2,5-diketopiperazines as rigid three-dimensional scaffolds in receptors and peptidomimetics. Eur. J. Org. Chem. 2011, 2, 217–228. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Sung, B.J.; Hwang, K.Y.; Jeon, Y.H.; Lee, J.I.; Heo, Y.S.; Kim, J.H.; Moon, J.; Yoon, J.M.; Hyun, Y.L.; Kim, E.; et al. Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature 2003, 425, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Liddle, J.; Allen, M.J.; Borthwick, A.D.; Brooks, D.P.; Davies, D.E.; Edwards, R.M.; Exall, A.M.; Hamlett, C.; Irving, W.R.; Mason, A.M.; et al. The discovery of GSK221149A: A potent and selective oxytocin antagonist. Bioorg. Med. Chem. Lett. 2008, 18, 90–94. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef]
- Mohanlal, R.W.; Lloyd, K.; Huang, L. Plinabulin, a novel small molecule clinical stage IO agent with anti-cancer activity, to prevent chemo–induced neutropenia and immune related AEs. J. Clin. Oncol. 2018, 36, 126. [Google Scholar] [CrossRef]
- Kanzaki, H.; Yanagisawa, S.; Kanoh, K.; Nitoda, T. A novel potent cell cycle inhibitor dehydrophenylahisti enzymatic synthesis and inhibitory activity towards eaurchin embryo. J. Antibiot. 2002, 55, 1042–1047. [Google Scholar] [CrossRef]
- Gu, B.; He, S.; Yan, X.; Zhang, L. Tentative biosynthetic pathways of some microbial diketopiperazines. Appl. Microbiol. Biotechnol. 2013, 97, 8439–8453. [Google Scholar] [CrossRef]
- Maeda, K.; Nakata, H.; Koh, Y.; Miyakawa, T.; Ogata, H.; Takaoka, Y. Spiro diketopiperazine-based CCR5 inhibitor whichpreservesCC-chemokine/CCR5 interactions and exerts potent activitya gainst R5 human immuno deficiency virus type 1 in vitro. J. Virol. 2004, 78, 8654–8662. [Google Scholar] [CrossRef]
- Sugie, Y.; Hirai, H.; Inagaki, T.; Ishiguro, M.; Kim, Y.J.; Kojima, Y. A new antibiotic CJ-17,665 from Aspergillusochraceus. J. Antibiot. 2001, 54, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Remko, M.; Breza, M.; Madura, I.D.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. A supramolecular approach to structure-based design with a focus on synthon hierarchy in ornithine-derived ligands: Review, synthesis, experimental and in silico studies. Molecules 2020, 25, 1135. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Stiles, D.T. Total synthesis of spirotryprostatin B via diastereoselective prenylation. Org. Lett. 2007, 9, 2763–2766. [Google Scholar] [CrossRef]
- Cui, C.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, cyclotroprostatins A–D, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1997, 53, 59–72. [Google Scholar] [CrossRef]
- Fabbri, D.; Adamiano, A.; Falini, G.; De Marco, R.; Mancini, I. Analytical pyrolysis of dipeptides containing proline and amino acids with polar side chains. Novel 2,5-diketopiperazine markers in the pyrolysates of proteins. J. Anal. Appl. Pyrolysis. 2012, 95, 145–155. [Google Scholar] [CrossRef]
- Xiang, W.X.; Liu, Q.; Li, X.M.; Lu, C.H.; Shen, Y.M. Four pairs of proline-containing cyclic dipeptides from Nocardiopsis sp. HT88, an endophytic bacterium of Mallotus nudiflorus L. Nat. Prod. Res. 2020, 34, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Begum Ahil, S.; Hira, K.; Shaik, A.B.; Pal, P.P.; Kulkarni, O.P.; Araya, H.; Fujimoto, Y. L-Proline-based-cyclic dipeptides from Pseudomonas sp. (ABS-36) inhibit pro-inflammatory cytokines and alleviate crystal-induced renal injury in mice. Int. Immunopharmacol. 2019, 73, 395–404. [Google Scholar] [CrossRef]
- Li, F.; Liu, K.; Gray, C.; Harris, P.; Reynolds, C.M.; Vickers, M.H.; Guan. Cyclic glycine-proline normalizes systolic blood pressure in high-fat diet-induced obese male rats. J. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 339–346. [Google Scholar]
- Turkez, H.; Cacciatore, I.; Arslan, M.E.; Fornasari, E.; Marinelli, L.; Di Stefano, A.; Mardinoglu, A. Histydyl-proline diketopiperazine isomers as multipotent anti-Alzheimer drug candidates. Biomolecules 2020, 10, 737–756. [Google Scholar] [CrossRef]
- Gomes, P.; Vale, R.; Moreira, R. Cyclization-activated prodrugs. Molecules 2007, 12, 2484–2506. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, H.; de Crecy-Lagard, V.; Zhu, W.; Richards, N.G.J.; Naismith, J.H. PMP-diketopiperazine adducts form at the active site of a PLP dependent enzyme involved in formycin biosynthesis. Chem. Commun. 2019, 55, 14502–14505. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, L.M.; Barry, S.M.; Rea, D.; Gallo, A.; Griffiths, D.; Lewandowski, J.R.; Fulop, V.; Challis, G.L. Binding of Distinct Substrate Conformations Enables Hydroxylation of Remote Sites in Thaxtomin D by Cytochrome P450 TxtC. J. Am. Chem. Soc. 2019, 141, 216–222. [Google Scholar] [CrossRef]
- Fonvielle, M.; Le Du, M.H.; Lequin, O.; Jacquet, M.; Thai, R.; Dubois, S.; Gondry, M.; Belin, P. Substrate and Reaction Specificity of Mycobacterium tuberculosis Cytochrome P450 CYP121: Insights from biochemical studies and crystal structures. J. Biol. Chem. 2013, 288, 17347–17359. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Li, W.B. Design, synthesis and biological activity evaluation of plinabulin derivatives based on co-crystal structure. Bioorganic & Medicinal Chemistry. 2018, 26, 2061–2072. [Google Scholar]
- Bourgeois, G.; Seguin, J.; Babin, M.; Belin, P.; Moutiez, M.; Mechulan, Y.; Gondry, M.; Schmitt, E. Structural basis for partition of the cyclodipeptide synthases into two subfamilies. J. Struct. Biol. 2019, 203, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Moutiez, M.; Schmitt, E.; Seguin, J.; Thai, R.; Favry, E.; Belin, P.; Mechulan, Y.; Gondry, M. Unravelling the mechanism of non-ribosomal peptide synthesis by cyclodipeptide synthases. Nat. Commun. 2014, 5, 5141–5141. [Google Scholar] [CrossRef]
- Schuller, J.M.; Zocher, G.; Liebhold, M.; Xie, X.; Stahl, M.; Li, S.M.; Stehle, T. Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J. Mol. Biol. 2012, 422, 87–99. [Google Scholar] [CrossRef]
- Bonnefond, L.; Arai, T.; Sakaguchi, Y.; Suzuki, T.; Ishitani, R.; Nureki, O. Structural basis for nonribosomal peptide synthesis by an aminoacyl-tRNA synthetase paralog. Proc. Natl. Acad. Sci. USA 2011, 108, 3912–3917. [Google Scholar] [CrossRef]
- Sauguet, L.; Moutiez, M.; Li, Y.; Belin, P.; Seguin, J.; Le Du, M.H.; Thai, R.; Masson, C.; Fonvielle, M.; Pernodet, J.L.; et al. Cyclodipeptide synthases, a family of class-I aminoacyl-tRNA synthetase-like enzymes involved in non-ribosomal peptide synthesis. Nucleic Acids. Res. 2011, 39, 4475–4489. [Google Scholar] [CrossRef]
- Cryle, M.J.; Bell, S.G.; Schlichting, I. Structural and biochemical characterization of the cytochrome P450 CypX (CYP134A1) from Bacillus subtilis: A cyclo-L-leucyl-L-leucyl dipeptide oxidase. Biochemistry 2010, 49, 7282–7296. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Wu, Y.J.; Chiang, Y.Y.; Kuo, C.Y.; Shrestha, K.L.; Chao, C.F.; Huang, Y.C.; Chuankhayan, P.; Wu, W.G.; Li, Y.K.; et al. Crystal structures of bacillus cereus NCTU2 chitinase complexes with chitooligomers reveal novel substrate binding for catalysis: A chitinase without chitin-binding and insertion domains. J. Biol. Chem. 2010, 285, 31603–31615. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Blanchard, J.S. The Structure and Mechanism of the Mycobacterium Tuberculosis Cyclodityrosine Synthetase. Nat. Chem. Biol. 2010, 6, 797. [Google Scholar] [CrossRef] [PubMed]
- Belin, P.; Le Du, M.H.; Fielding, A.; Lequin, O.; Jacquet, M.; Charbonnier, J.B.; Lecoq, A.; Thai, R.; Courcon, M.; Masson, C.; et al. Identification and structural basis of the reaction catalyzed by CYP121, an essential cytochrome P450 in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2009, 106, 7426–7431. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.R.; Synstad, B.; Ejsink, V.G.H.; Stark, M.J.; Eggleston, I.; Van Aalten, D.M.F. Structure-Based Exploration of Cyclic Dipeptide Chitinase Inhibitors. J. Med. Chem. 2004, 47, 5713. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Luo, Z.; Zhang, W.; Deng, Z.; Mobli, M.; Kobe, B.; Jia, X.; Qu, X. Molecular Basis of Regio- and Stereo-Specificity in Biosynthesis of Bacterial Heterodimeric Diketopiperazines. Nature Commun. 2020, 11, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.R.; Eggleston, I.; Synstad, B.; Ejsink, V.G.; van Aalten, D.M. The cyclic dipeptide CI-4 [cyclo-(l-Arg-d-Pro)] inhibits family 18 chitinases by structural mimicry of a reaction intermediate. Biochem. J. 2002, 368, 23–27. [Google Scholar] [CrossRef]
- Classen, S.; Olland, S.; Berger, J.M. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc. Natl. Acad. Sci. USA 2003, 100, 10629–10634. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Cryst. B 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Cole, J.C.; Wiggin, S.; Stanzione, F. New insights and innovation from a million crystal structures in the Cambridge Structural Database. Struc. Dyn. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Groom, C.R.; Cole, J.C. The use of small-molecule structures to complement protein-ligand crystal structures in drug discovery. Acta Cryst. D 2017, 73, 240–245. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarska, J.; Wolf, W.M. Ultra-Short Cyclo-Peptides as Bio-Inspired Therapeutics: Proline-Based 2,5-Diketopiperazines (DKP). Proceedings 2021, 79, 10. https://doi.org/10.3390/IECBM2020-08804
Bojarska J, Wolf WM. Ultra-Short Cyclo-Peptides as Bio-Inspired Therapeutics: Proline-Based 2,5-Diketopiperazines (DKP). Proceedings. 2021; 79(1):10. https://doi.org/10.3390/IECBM2020-08804
Chicago/Turabian StyleBojarska, Joanna, and Wojciech M. Wolf. 2021. "Ultra-Short Cyclo-Peptides as Bio-Inspired Therapeutics: Proline-Based 2,5-Diketopiperazines (DKP)" Proceedings 79, no. 1: 10. https://doi.org/10.3390/IECBM2020-08804
APA StyleBojarska, J., & Wolf, W. M. (2021). Ultra-Short Cyclo-Peptides as Bio-Inspired Therapeutics: Proline-Based 2,5-Diketopiperazines (DKP). Proceedings, 79(1), 10. https://doi.org/10.3390/IECBM2020-08804




