Abstract
Glycolysis inhibitors are currently considered as potential anticancer agents due to the fact that significant changes occur in tumor cells, known as metabolic reprogramming. However, carbohydrate metabolism inhibitors have pronounced systemic toxicity. Using liposomal forms of drugs as targeted delivery is a way to decrease systemic toxicity. Using high-performance liquid chromatography, the optimal conditions for obtaining iodoacetate encapsulated in liposomes and its final concentration in them were established. Based on the indicators of biochemical markers of the toxic effect of iodoacetate encapsulated in liposomes, it was found that when administered in a course for two weeks, iodoacetate in this form has neither cardiotoxic nor any other negative effects on target organs, that is, it is safe.
1. Introduction
Discovery and further studies of metabolic reprogramming of tumor cells have stimulated the search for new antitumor antimetabolites. Currently, numerous antimetabolite inhibitors demonstrating pronounced antitumor effect are tested in clinical trials that will open up prospects for the development of new approaches for cancer therapy that could potentially change the uncontrolled course of disease [1]. Today, the search for new anticancer agents is widely represented by the class of antimetabolites. Among them, glycolysis inhibitors have the most effective antitumor properties. However, most of them have pronounced systemic toxicity [2]. The development of modified antitumor agents based on glycolysis inhibitors using methods of targeted drug delivery is urgent. One of the approaches to reduce the systemic toxic effect and increase the therapeutic index is targeted drug delivery using liposomes [3]. Among the inhibitors of glycolysis, one can distinguish such an inhibitor as iodoacetate, which has antitumor activity [4], but has a cardiotoxic effect [5], which can be significantly reduced using the liposomal form of the drug.
The aim of the study was to develop a method for obtaining liposomes of various diameters for iodoacetate and to evaluate their systemic toxic effect.
2. Materials and Methods
For the analysis of liposomes, we used the conditions for chromatographic separation of phospholipids, cholesterol, and iodoacetate, using aminopropyl silica gel as the stationary phase and acetonitrile solution (ACS, Panreac, Spain) with water in a 80:20 ratio as the mobile phase. Maintaining the optimal pH in the mobile phase was carried out by adding acetic acid (content in PP—60 mmol/L) and ammonium formate (content in PP—30 mmol/L). Iodoacetate was detected spectrophotometrically at a wavelength of λ = 260 nm. The chromatographic system ensures the separation of phospholipids, cholesterol and compounds under study in real samples (dosage forms with liposomes with a diameter of 100 nm and 400 nm). The technique provided repeatability, intermediate precision, correctness and sensitivity at a sufficient level.
We used 1,2-dipalmitoyl-glycero-3-phosphocholine (DPPC) and cholesterol (Chol) (Sigma Aldrich, city, Japan) to obtain a hollow liposome emulsion. Lipids were dissolved in chloroform, after which a lipid film was formed on a rotary evaporator at 56 °C. Molar ratios of lipids: DPPC: Chol = 9:0.02. The emulsion and suspension formed after the hydration of lipid films were transferred into the working chamber of the extruder and filtered through polycarbonate filters with a diameter of 100 nm and 400 nm (Sartorius, Göttingen, Germany). Glycolysis inhibitor iodoacetate (Sigma-Aldrich, Darmstadt, Germany) was placed in an extruder chamber containing a liposome emulsion. At a temperature of 45–60 °C, the mixture was filtered 11 times through a membrane under argon pressure in the range from 2 to 10 MPa. The resulting liposomes were purified from the unincorporated component using a dialysis membrane (Carl roth gmbh, Karlsruhe, Germany).
The assessment of the toxic effect was carried out on mice of the C57BL/6j line (males) weighing 28–30 g, obtained from the nursery of the Goldberg Research Institute of Pharmacology and Regenerative Medicine (Tomsk, Russia). The studies were carried out in compliance with the rules of laboratory practice during preclinical research in the Russian Federation (GOST R 51000.3 96 and GOST R 51000.4 96), international recommendations of the “European Convention for the Protection of Vertebrate Animals Used for Experiments or Other Scientific Purposes” (Strasburg, 1986).
Vein catheterization in animals was carried out 2 days before the start of blood sampling in accordance with the protocol [6]. The operation was performed under Isoflurane (Baxter, Deerfield, IL, USA) anesthesia using an animal anesthesia station (Braintree scientific, EZ-7000-320, Braintree, MA, USA). For intravenous administration, a 27G catheter (Sci-cat, city, Russia) was used.
Blood was obtained from the jugular vein. Blood was subsequently collected in tubes prefilled with heparin. Blood was collected every third day for 12 days.
Heparin-anticoagulated plasma was obtained following 10 min of centrifugation at 2000 rcf and analyzed for clinical chemistry parameters on an automatic biochemical analyzer Architect (Abbot, Lake Forest, IL, USA). For the study of total protein, creatinine, glucose, bilirubin, enzymatic activity of ALAT, ASAT, LDH, alkaline phosphatase, standard methods were used according to the manufacturers’ protocols.
Statistical data processing was performed using the Statistica 6.0 software package.
3. Results and Discussion
3.1. Chromatographic Studies of Liposomal forms of Iodoacetate
Evaluation of the optimal conditions for obtaining liposomes showed that the preparation of liposomes by extrusion at moderate temperatures (35–50 °C) is possible using membranes with pores of 400 nm. Moreover, the pressure required to obtain them does not exceed 3 MPa. In the case of using pores with a small size (100 nm), it is necessary to use a higher temperature (60 °C) and a pressure of 10 MPa. Further studies using high-performance liquid chromatography (HPLS) showed that such conditions during the formation of liposomes lead to large losses of low molecular weight compounds.
When choosing the conditions for preparing a sample for analysis by HPLC, two aspects had to be taken into account. First, to assess the quantitative content of agents directly in liposomes, it was necessary to select a method for separating these liposomes from the interliposomal fluid, since it will also contain some part of the drug, and when working with real dosage forms, it will be necessary to subtract from the total amount of the drug determined in the dosage form.
Among the approaches to solving a really nontrivial problem, several options were considered: separation by centrifugation, separation by filtration, and separation by sedimentation. The first two options have proven to be very ineffective. Thus, when centrifuging liposomal dosage forms under different conditions, the emulsion did not separate into two layers: the liposomes remained evenly distributed in the volume of the interliposomal fluid. Separation by filtration also did not fit as a method of sample preparation, since the use of filters will lead to the rupture of liposomes, and, as a consequence, release of the active substance from them.
Activated carbon deposition has proven to be an effective separation method. The use of an emulsion of liposomes together with activated carbon after freezing ensures separation of the solution in two layers: interliposomal liquid—the upper transparent layer, activated carbon by adsorbed liposomes—a precipitated layer with liposome clots.
Secondly, the task was set to find an alternative to existing agents that promote more efficient liposome opening. The first and one of the most common ways to open liposomes is to use surfactants. Among the surfactants mentioned in published studies that are used for these purposes are Triton X, Nonidet R-40.
After analyzing each of the various options, it turned out that the most effective way to open liposomes can be considered the use of aprotic solvents (Table 1).

Table 1.
Comparative evaluation of the effectiveness of agents that destroy the membrane of liposomes.
According to the selected method, samples of liposomal dosage forms containing sodium iodoacetate with liposome diameters of 100 and 400 nm were analyzed. The results of the analysis according to the developed method made it possible to evaluate the efficiency of liposome creation by the ratio of the amount of active substance “hardwired” in them and the amount of iodoacetate lost during the creation of liposomes.
According to the results of the analysis of the samples, it was found that the dialysis of liposomes from the non-included drug is 3 h.
Examples of chromatograms of liposomes with iodoacetate, obtained during the analysis of dialysis samples, are shown in Figure 1 and Figure 2.
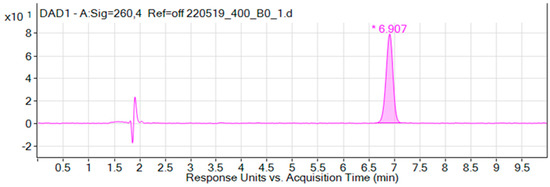
Figure 1.
Chromatogram of the liposomal form of iodoacetate (sample without dialysis). The chromatogram shows the peak of iodoacetate at 7 min. Liposome components are detected in 2 min.
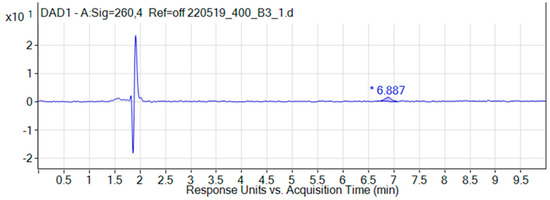
Figure 2.
Chromatogram of the liposomal dosage form of iodoacetate (dialysis 3 h).
Chromatographic studies of the amount of incorporated iodoacetate in liposomes showed that at a size of both 100 nm and 400 nm and a liposome concentration of 8–10 mg/mL, the limiting concentration of the drug inclusion was close to the MTD for animals with a grafted tumor and was about 9 mg/kg.
This indicates that the assessment of the protective function of encapsulation in liposomes will be determined by the frequency of drug administration. An increase in the concentration of liposomes in solution is not permissible, since the study used the maximum permissible concentration for intravenous administration. An increase in the lipid content of the solution may increase the risks of vascular thrombosis.
3.2. Biochemical Analysis of Animal Blood after Course Administration of Iodoacetate
Analysis of biochemical parameters of blood showing the functional state of the main target organs revealed that in an independent form, iodoacetate has mainly a toxic effect on the heart and pancreas. Thus, the ASAT level increased to 750 U/L with a normal value of 50 U/L, and the LDH activity exceeded 1500 U/L, which is more than three times higher than the norm. The content of total amylase in the blood also during intoxication with iodine acetate exceeded normal values almost three times and was, on average, about 2000 U/L. Against the background of these shifts, the glucose level increased to 16 mmol/L, while the normal values in animals were 9.6 mmol/L. However, the most significant effect of iodoacetate was associated with the effect on the liver. We found that the inhibitor does not have any hepatotoxic effect, against the background of an increase in other biochemical markers of toxic effect by two to three times relative to normal values. So, the ALAT level did not go beyond the normal range and amounted to 16 U/L.
The study of the action of iodoacetate in liposomal form, both with a single and double administration, did not reveal any deviations from the norm of any biochemical marker. This result is expected, since it is known that the distribution of unmodified liposomes is almost completely concentrated in the liver, spleen, and tumors [7].
Thus, the liposomal form of iodoacetate, having a safe profile in contrast to its independent form, has great potential in anticancer therapy, especially in polypharmacy.
4. Conclusions
The glycolysis inhibitor iodoacetate causes significant disturbances in carbohydrate metabolism, which ultimately leads to serious heart damage. In this case, the inhibitor does not show toxic effects on the liver. The liposomal form of iodoacetate, due to the peculiarities of their biodistribution and the absence of hepatotoxic action in the agent, has shown itself to be a safe dosage form. This makes iodoacetate encapsulated in liposomes a promising compound for further research as an anticancer agent.
5. Patents
Korshunov, D.A.; Klimov, I.A. Anti-tumor liposomal drug and method of its preparation. RU 2 663 291 C1.
Institutional Review Board Statement
In this section, you should add the Institutional Review Board Statement and approval number, if relevant to your study. You might choose to exclude this statement if the study did not require ethical approval. Please note that the Editorial Office might ask you for further information. Please add “The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Cancer Research Institute Tomsk NRMC (protocol date of approval 30.10.2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korshunov, D.A.; Kondakova, I.V.; Shashova, E.E. Modern Perspective on Metabolic Reprogramming in Malignant Neoplasms. Biochemistry 2019, 84, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Nielson, T.C.; Le, H.V. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr. Top. Med. Chem. 2018, 18, 494–504. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives. Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, D.A.; Klimov, I.A.; Ivanov, V.V.; Kondakova, I.V. Glycolysis Inhibitors Monoiodoacetate and 2-Deoxyglucose as Antitumor Agents: Experimental Study on Lewis Lung Carcinoma Model. Bull. Exp. Biol. Med. 2018, 165, 695–697. [Google Scholar] [CrossRef]
- Abe, H. Regulation of Cardiac Function: Molecular, Cellular and Pathophysiological Aspects; Japan Scientific Societies Press: Tokyo, Japan, 1984; p. 330. [Google Scholar]
- Kmiotek, E.K.; Baimel, C.; Gill, K.J. Methods for Intravenous Self Administration in a Mouse Model. J. Vis. Exp. 2012, 70, e3739. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.J.; De Rosales, R.T.M. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).