Key Features of Solid Lipid Nanoparticles Prepared with Nanoclay and Spring Water Ingredients with Demonstrated Wound Healing Activity: A Pilot Study †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Formulation Procedure
2.2.2. Characterization of the Prepared Formulations
3. Results and Discussion
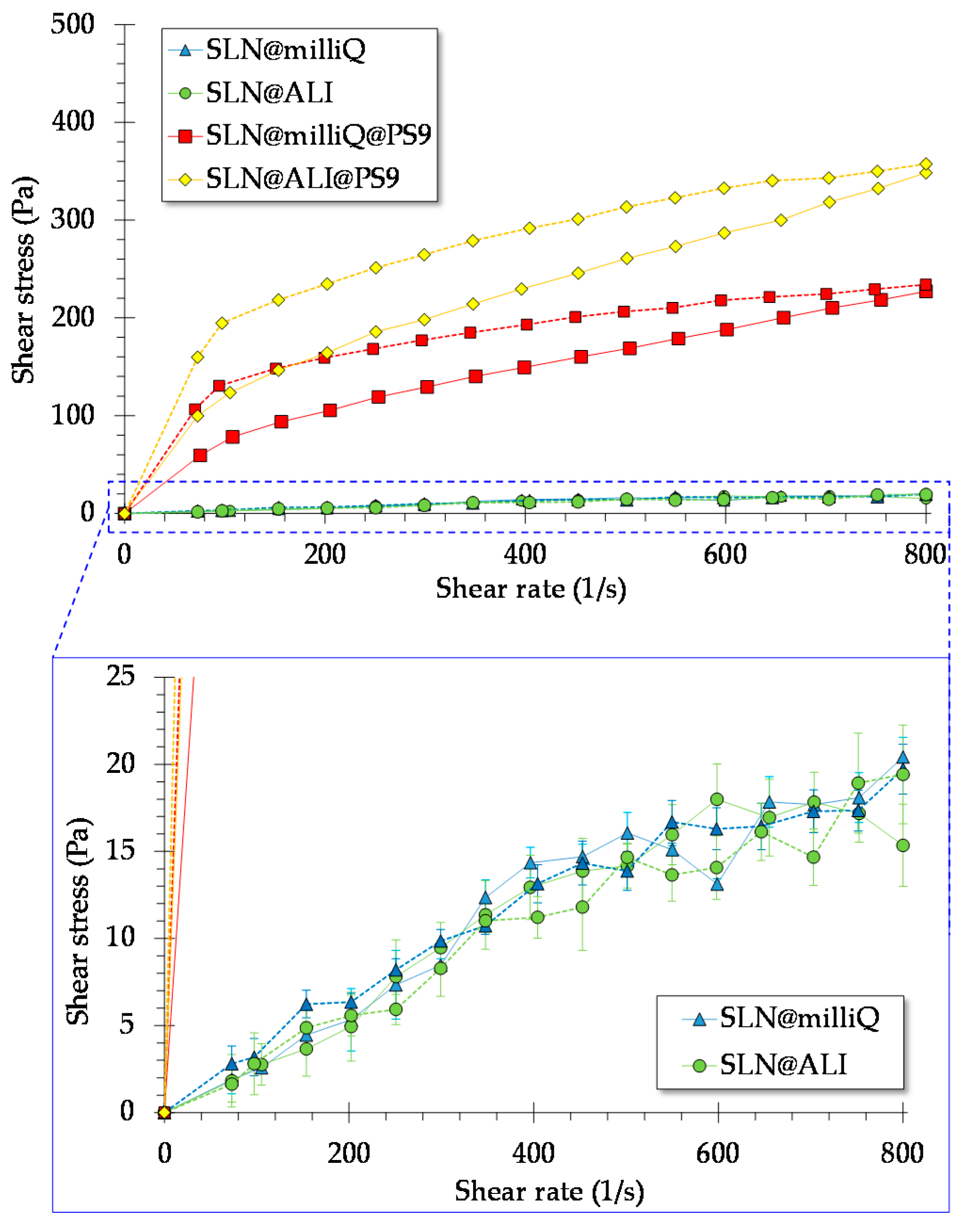
3.1. Rheology Studies
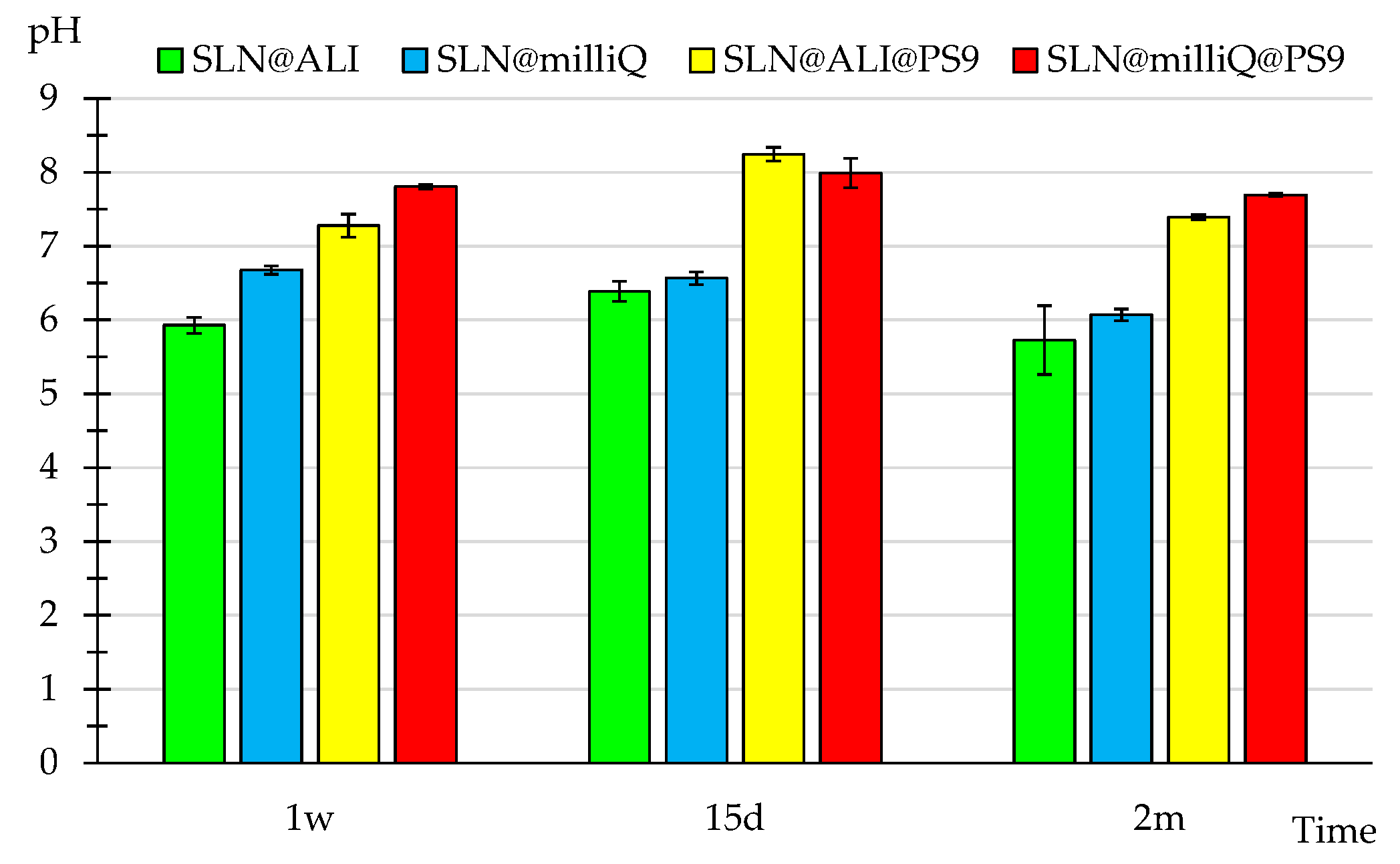
3.2. pH
3.3. Particle Size
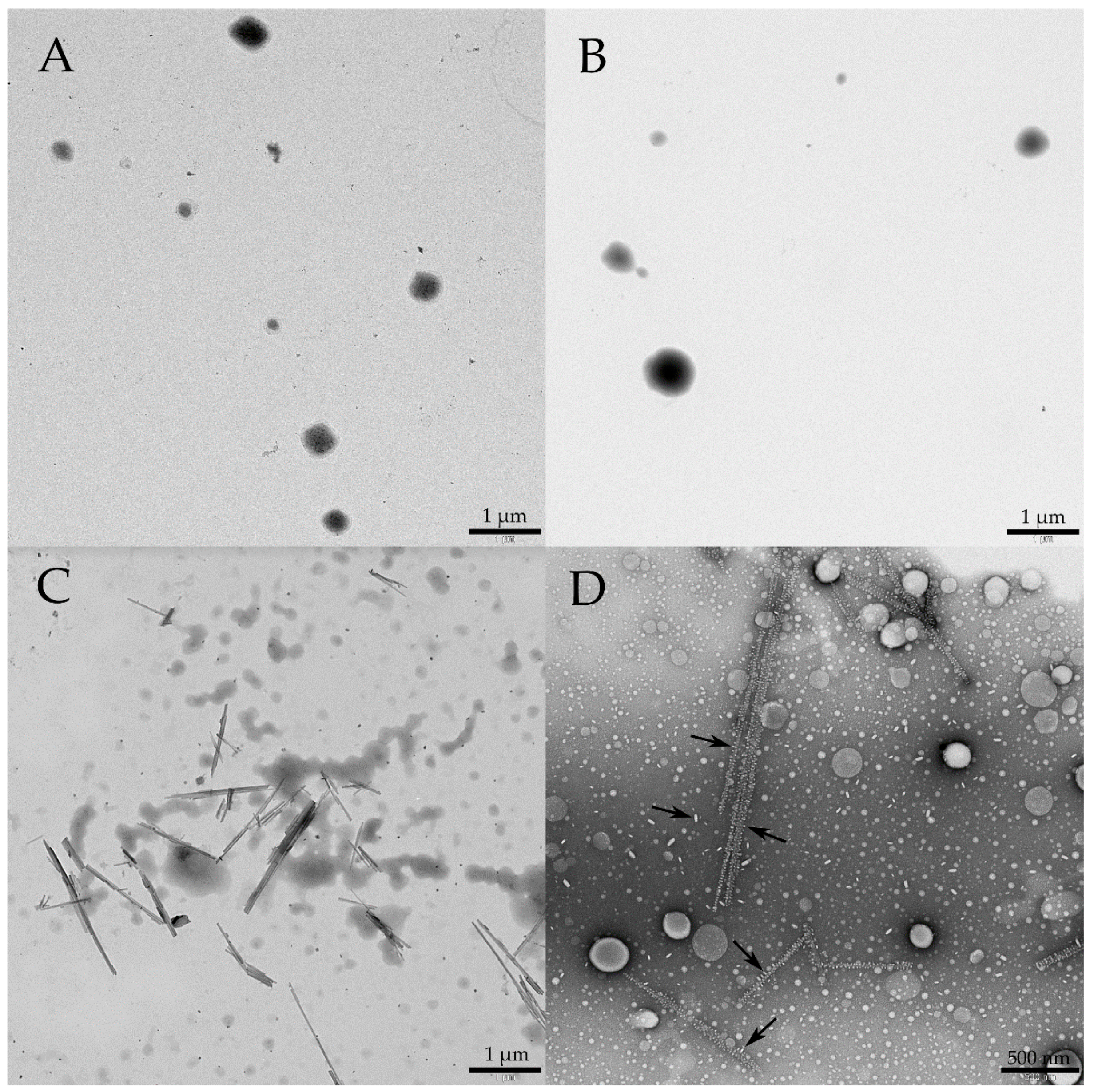
3.4. TEM
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.M.; Da Silva, C.M.G.; Bella, T.S.; De Araújo, D.R.; Marcato, P.D.; Durán, N.; De Paula, E. Cytotoxicity of solid lipid nanoparticles and nanostructured lipid carriers containing the local anesthetic dibucaine designed for topical application. J. Phys. Conf. Ser. 2013, 429. [Google Scholar] [CrossRef]
- De Araújo, D.R.; Da Silva, D.C.; Barbosa, R.M.; Franz-Montan, M.; Cereda, C.M.; Padula, C.; Santi, P.; De Paula, E. Strategies for delivering local anesthetics to the skin: Focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin. Drug Deliv. 2013, 10, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Sarecka-Hujar, B.; Banyś, A.; Ostróżka-Cieślik, A.; Balwierz, R.; Dolińska, B. Evaluation of the potential of nanoparticles containing active substances in selected chronic diseases. Adv. Clin. Exp. Med. 2020, 29, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Romić, M.D.; Sušac, A.; Lovrić, J.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Evaluation of stability and in vitro wound healing potential of melatonin loaded (lipid enriched) chitosan based microspheres. Acta Pharm. 2019, 69, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Abd El-Rahman, F.A.A.; Hamdy, G.M. Chamomile oil loaded solid lipid nanoparticles: A naturally formulated remedy to enhance the wound healing. J. Drug Deliv. Sci. Technol. 2019, 50, 329–338. [Google Scholar] [CrossRef]
- Rosseto, H.C.; de Toledo, L.A.S.; de Francisco, L.M.B.; de Esposito, E.; Lim, Y.; Valacchi, G.; Cortesi, R.; Bruschi, M.L. Nanostructured lipid systems modified with waste material of propolis for wound healing: Design, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ramasamy, K.; Lim, S.M.; Ismail, M.F.; Majeed, A.B.A. Antimicrobial and in vitro wound healing properties of novel clay based bionanocomposite films. J. Mater. Sci. Mater. Med. 2014, 25, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Girbau, C.; Alonso, R.; Aguirre, J.J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. LL37 loaded nanostructured lipid carriers (NLC): A new strategy for the topical treatment of chronic wounds. Eur. J. Pharm. Biopharm. 2016, 108, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; D’Autilia, F.; Rossi, S.; Ferrari, F.; Grisoli, P.; Sorrenti, M.; Catenacci, L.; Del Fante, C.; Perotti, C.; et al. Wound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairing. Eur. J. Pharm. Biopharm. 2013, 84, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Salado, C.; Vega, S.; Aizpurua-Olaizola, O.; Arada, I.; de la Suarez, T.; Usobiaga, A.; Arrondo, J.L.R.; Alonso, A.; Goñi, F.M.; et al. Solid lipid nanoparticles for delivery of Calendula officinalis extract. Colloids Surf. B Biointerfaces 2015, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- De Vringer, T.; De Ronde, H.A.G. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. J. Pharm. Sci. 1995, 84, 466–472. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Souza, I.M.S.; de Melo Barbosa, R.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Ojeda-Riascos, S.; Viseras, C. Natural inorganic ingredients in wound healing. Curr. Pharm. Des. 2020, 26, 621–641. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Faccendini, A.; Miele, D.; Ruggeri, M.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Rossi, S.; Viseras, C.; Sandri, G. Wound healing activity of nanoclay/spring water hydrogels. Pharmaceutics 2020, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Cucca, L.; Sandri, G.; Viseras, C. Correlation between elemental composition/mobility and skin cell proliferation of fibrous nanoclay/spring water hydrogels. Pharmaceutics 2020, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Perioli, L.; Viseras, C. Safety of nanoclay/spring water hydrogels: Assessment and mobility of hazardous elements. Pharmaceutics 2020, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Sánchez-Espejo, R.; López-Galindo, A.; Cerezo, P.; Viseras, C. Design and characterization of spring water hydrogels with natural inorganic excipients. Appl. Clay Sci. 2020, 197, 105772. [Google Scholar] [CrossRef]
- Prado-Pérez, A.J.; Pérez del Villar, L. Dedolomitization as an analogue process for assessing the long-term behaviour of a CO2 deep geological storage: The Alicún de las Torres thermal system (Betic Cordillera, Spain). Chem. Geol. 2011, 289, 98–113. [Google Scholar] [CrossRef]

| Sample | d10 (μm) | d50 (μm) | d90 (μm) | SPAN Factor |
|---|---|---|---|---|
| SLN@milliQ_1w | 0.073 | 0.124 | 0.202 | 1.041 |
| SLN@ALI_1w | 0.074 | 0.124 | 0.203 | 1.042 |
| SLN@milliQ@PS9_1w | 0.116 | 1.595 | 8.277 | 5.116 |
| SLN@ALI@PS9_1w | 0.141 | 1.776 | 7.747 | 4.283 |
| SLN@ALI@PS9_2m | 0.180 | 1.235 | 7.650 | 6.046 |
| SLN@milliQ@PS9_2m | 0.142 | 1.435 | 6.143 | 4.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villén, F.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Barbosa, R.d.M.; Viseras, C. Key Features of Solid Lipid Nanoparticles Prepared with Nanoclay and Spring Water Ingredients with Demonstrated Wound Healing Activity: A Pilot Study. Proceedings 2021, 78, 56. https://doi.org/10.3390/IECP2020-08694
García-Villén F, Sánchez-Espejo R, Borrego-Sánchez A, Cerezo P, Barbosa RdM, Viseras C. Key Features of Solid Lipid Nanoparticles Prepared with Nanoclay and Spring Water Ingredients with Demonstrated Wound Healing Activity: A Pilot Study. Proceedings. 2021; 78(1):56. https://doi.org/10.3390/IECP2020-08694
Chicago/Turabian StyleGarcía-Villén, Fátima, Rita Sánchez-Espejo, Ana Borrego-Sánchez, Pilar Cerezo, Raquel de Melo Barbosa, and César Viseras. 2021. "Key Features of Solid Lipid Nanoparticles Prepared with Nanoclay and Spring Water Ingredients with Demonstrated Wound Healing Activity: A Pilot Study" Proceedings 78, no. 1: 56. https://doi.org/10.3390/IECP2020-08694
APA StyleGarcía-Villén, F., Sánchez-Espejo, R., Borrego-Sánchez, A., Cerezo, P., Barbosa, R. d. M., & Viseras, C. (2021). Key Features of Solid Lipid Nanoparticles Prepared with Nanoclay and Spring Water Ingredients with Demonstrated Wound Healing Activity: A Pilot Study. Proceedings, 78(1), 56. https://doi.org/10.3390/IECP2020-08694






