Release of Ropinirole from Acrylate-Vinylacetate Transdermal Formulations: Modulation Based on Polymer-Drug Interactions †
Abstract
:1. Introduction
2. Material & Methods
2.1. Experimental
2.1.1. Preparation of Formulations
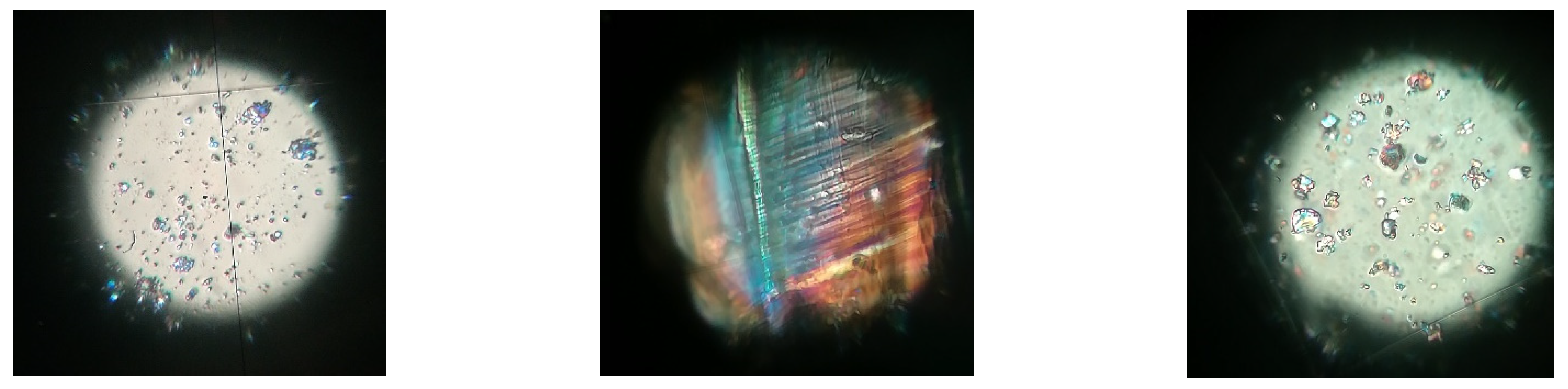
2.1.2. Optical Microscopy
2.1.3. Drug Release
2.2. Release Parameters
2.2.1. Descriptive and Explicative Parameters
2.2.2. Model Selection
2.2.3. Comparison of Release Parameters
3. Results
3.1. Solubility Data
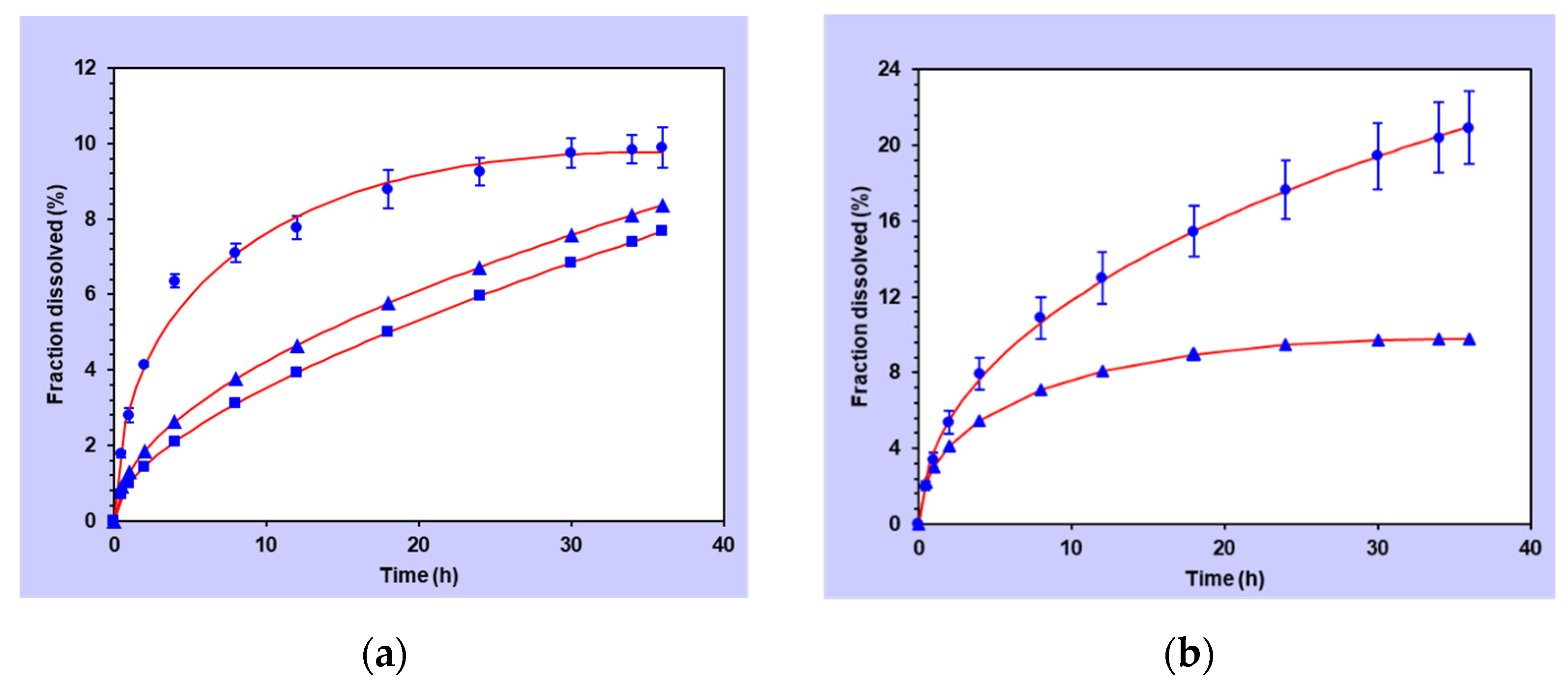
3.2. Release Results
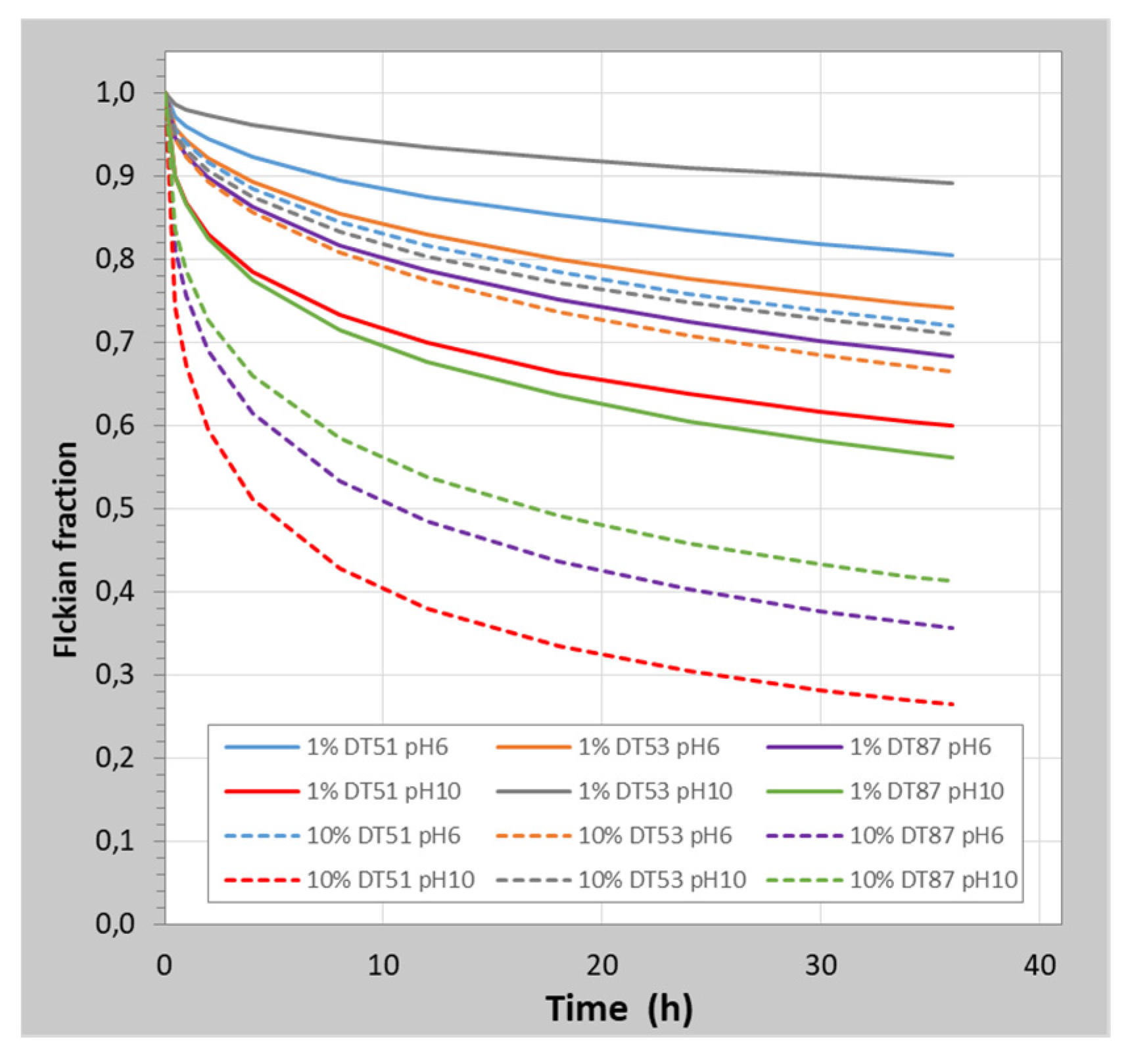
Release Parameters
4. Discussion
4.1. Drug Solubilisation
4.2. Effect of Formulation Variables on Ropinirole Release
4.3. Descriptive Equations of Ropinirole Release from the Formulations
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Alfred, F.; Scherphof, G. Voigt’s Pharmaceutical Technology; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-118-97262-5. [Google Scholar]
- Sachdeva, V.; Bai, Y.; Kydonieus, A.; Banga, A.K. Formulation and optimization of desogestrel transdermal contraceptive patch using crystallization studies. Int. J. Pharm. 2013, 441, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jenquin, M.R.; McGinity, J.W. Characterization of acrylic resin matrix films and mechanisms of drug-polymer interactions. Int. J. Pharm. 1994, 101, 23–34. [Google Scholar] [CrossRef]
- Drug-In-Polymer Solubility Calculator for DURO-TAK Adhesives. Available online: http://calculator.duro-tak.com/ (accessed on 20 November 2019).
- Drugbank Database. Available online: www.drugbank.com (accessed on 13 June 2018).
- Soler, L.I.; Boix, A.; Lauroba, J.; Colom, H.; Domenech, J. Transdermal delivery of alprazolam from a monolithic patch: Formulation based on in vitro characterization. Drug Dev. Ind. Pharm. 2012, 38, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Jafri, I.; Shoaib, M.H.; Yousuf, R.I.; Ali, F.R. Effect of permeation enhancers on in vitro release and transdermal delivery of lamotrigine from Eudragit®RS100 polymer matrix-type drug in adhesive patches. Prog. Biomater. 2019, 8, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Magos, L.C.; Lentner, C. (Eds.) Geigy Scientific Tables, 8th ed.; Ciba-Geigy: Basel, Switzerland, 1986; p. 330. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Evaluation of mathematical models describing drug release from estradiol transdermal systems. Drug Dev. Ind. Pharm. 2003, 29, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Costa, F.O.; Sousa, J.J.S.; Pais, A.A.C.C.; Formosinho, S.J. Comparison of dissolution profiles of Ibuprofen pellets. J. Control. Release 2003, 89, 199–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ameen, D.; Michniak-Kohn, B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: Effect of penetration enhancers and crystallization inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef] [PubMed]
| Polymer | Drug Concentration | pH | AUCq(36 h) | SDAUC | Q24 | SDQ24 |
|---|---|---|---|---|---|---|
| DT51 | 1% | 6 | 4010.31 | 461.97 | 7.25% | 0.65 |
| 5% | 6 | 4257.70 | 2263.93 | 1.37% | 0.70 | |
| 10% | 6 | 6519.53 | 1202.29 | 0.92% | 0.24 | |
| DT51 | 1% | 10 | 4250.77 | 1334.82 | 5.66% | 1.35 |
| 5% | 10 | 5096.31 | 998.21 | 1.88% | 0.43 | |
| 10% | 10 | 9797.59 | 1113,25 | 1.67% | 0.28 | |
| DT53 | 1% | 6 | 2465.69 | 240.98 | 6.08% | 0.92 |
| 5% | 6 | 13,838.46 | 1100.00 | 6.11% | 0.27 | |
| 10% | 6 | 45,965.68 | 2346.86 | 9.26% | 0.35 | |
| DT53 | 1% | 10 | 6439.40 | 1672.07 | 14.45% | 2.81 |
| 5% | 10 | 26,764.40 | 1395.65 | 12.79% | 0.85 | |
| 10% | 10 | 79,751.50 | 3268.24 | 17.66% | 1.55 | |
| DT87 | 1% | 6 | 4470.79 | 1718.34 | 9.27% | 2.76 |
| 5% | 6 | 13,205.45 | 646.84 | 5.36% | 0.64 | |
| 10% | 6 | 15,313.37 | 4873.60 | 3.83% | 1.65 | |
| DT87 | 1% | 10 | 4151.08 | 835.52 | 8.63% | 1.06 |
| 5% | 10 | 12,706.81 | 1134.98 | 5.64% | 1.49 | |
| 10% | 10 | 13,022.45 | 3014.94 | 2.75% | 0.80 |
| Polymer | AUCq | Q24 | ||||
|---|---|---|---|---|---|---|
| 1% | 5% | 10% | 1% | 5% | 10% | |
| DT51 | 7.45 × 101 | 5.23 × 101 | 7.11 × 103 | 1.04 × 101 | 2.63 × 101 | 6.79 × 103 |
| DT53 | 3.31 × 103 | 6.62 × 106 | 2.85 × 106 | 1.31 × 103 | 5.51 × 106 | 4.26 × 105 |
| DT87 | 7.49 × 101 | 4.74× 101 | 4.54 × 101 | 6.80 × 101 | 7.39 × 101 | 2.84 × 101 |
| Polymer | AUCq | Q24 | ||
|---|---|---|---|---|
| pH6 | pH10 | pH6 | pH10 | |
| DT51 | 8.03 × 102 | 1.65 × 104 | 1.10 × 104 | 1.27 × 104 |
| DT53 | 4.29 × 1011 | 1.66 × 1011 | 5.78 × 105 | 1.67 × 102 |
| DT87 | 1.49 × 103 | 1.48 × 104 | 7.63 × 103 | 1.83 × 104 |
| Polymer | pH6 | pH10 | ||||
|---|---|---|---|---|---|---|
| 1% | 5% | 10% | 1% | 5% | 10% | |
| DT51 | HF0 | PS | HF0 | HF0 | PS | PS |
| DT53 | HF0 | PS | PS | PS | PS | PS |
| DT87 | PS | H | PS | PS | H | PS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterna-Paterna, J.; Miñarro-Carmona, M.; Ticó-Grau, J.R.; Boix-Montañés, A. Release of Ropinirole from Acrylate-Vinylacetate Transdermal Formulations: Modulation Based on Polymer-Drug Interactions. Proceedings 2021, 78, 38. https://doi.org/10.3390/IECP2020-08676
Paterna-Paterna J, Miñarro-Carmona M, Ticó-Grau JR, Boix-Montañés A. Release of Ropinirole from Acrylate-Vinylacetate Transdermal Formulations: Modulation Based on Polymer-Drug Interactions. Proceedings. 2021; 78(1):38. https://doi.org/10.3390/IECP2020-08676
Chicago/Turabian StylePaterna-Paterna, Jesús, Montserrat Miñarro-Carmona, Josep Ramon Ticó-Grau, and Antonio Boix-Montañés. 2021. "Release of Ropinirole from Acrylate-Vinylacetate Transdermal Formulations: Modulation Based on Polymer-Drug Interactions" Proceedings 78, no. 1: 38. https://doi.org/10.3390/IECP2020-08676
APA StylePaterna-Paterna, J., Miñarro-Carmona, M., Ticó-Grau, J. R., & Boix-Montañés, A. (2021). Release of Ropinirole from Acrylate-Vinylacetate Transdermal Formulations: Modulation Based on Polymer-Drug Interactions. Proceedings, 78(1), 38. https://doi.org/10.3390/IECP2020-08676





