Ex Vivo and In Vivo Anti-inflammatory Evaluations of Modulated Flavanones Solutions †
Abstract
1. Introduction
2. Experiments
2.1. Extraction and Isolation of Plant Material
2.2. Semi-synthesis from Natural Prenylated Flavanone
2.3. Anti-inflammatory Testing
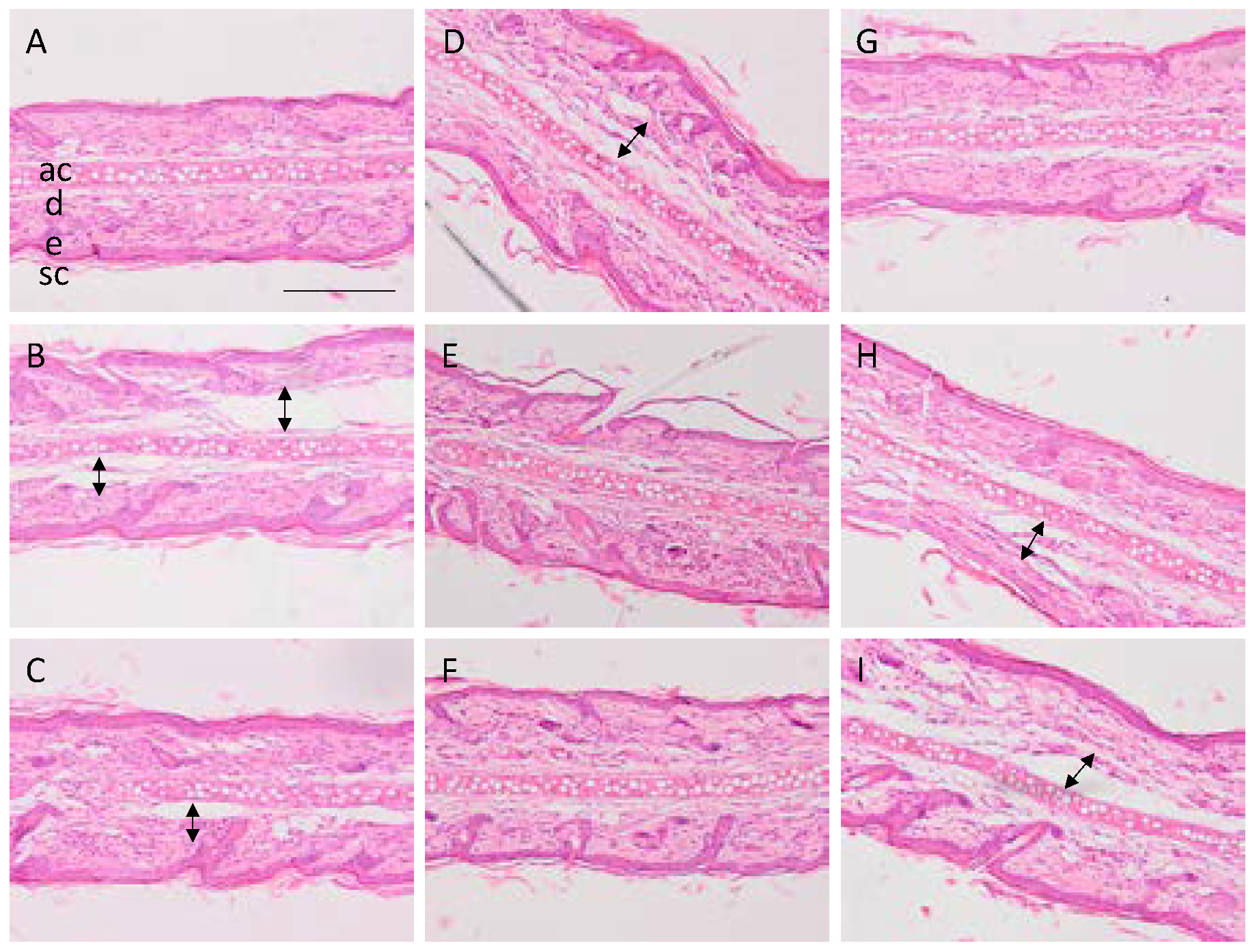
2.4. Histological Analysis
3. Results
3.1. Model of Mice Ear Inflammation Induced with TPA
3.2. In Vivo Rat Model and Anti-inflammatory Response after Flavanone Solutions Treatment
3.3. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| FS | Flavanone solution |
| nFS | Solution without any flavanone |
| TLC | Thin-layer chromatography |
| NSAID | Non-steroidal anti-inflammatory drugs |
| MeOH | Methanol |
| 1H-NMR | Proton nuclear magnetic resonance |
| ref | Reference drug |
| EtOH | Ethanol |
| H2O | Water |
| PBS | Phosphate buffered saline |
| SCH | Stratum corneum hydration |
| AU | Arbitrary units |
| PMN | Polymorphonuclear |
| COX | Cyclooxygenase |
| LOX | Lipooxigenase |
| SAR | Structure activity relationship |
| DNA | Deoxyribonucleic Acid |
| PCK | protein kinase C |
| MAPKs | mitogen activated protein kinases |
| PLA2 | phospholipase A2 |
References
- Abdel-Mottaleb, M.M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine strategies for targeting skin inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur. J. Pharmacol. 2009, 620, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Nag, M.K.; Patel, S.; Daharwal, S.J. Novel Approaches for Dermal and Transdermal Delivery of Herbal Drugs. Res. J. Pharmacogn. Phytochem. 2013, 5, 271–279. [Google Scholar]
- Alalaiwe, A.; Lin, C.-F.; Hsiao, C.-Y.; Chen, E.-L.; Lin, C.-Y.; Lien, W.-C.; Fang, J.-Y. Development of flavanone and its derivatives as topical agents against psoriasis: The prediction of therapeutic efficiency through skin permeation evaluation and cell-based assay. Int. J. Pharm. 2020, 581, 119256. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Feng, X.E.; Cui, J.R.; Fang, L.; Du, G.H.; Li, Q.S. Synthesis and biological activity of flavanone derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 5466–5468. [Google Scholar] [CrossRef]
- Narváez-Mastache, J.M.; Soto, C.; Delgado, G. Antioxidant Evaluation of Eysenhardtia Species (Fabaceae): Relay Synthesis of 3-O-Acetyl-11α,12α-epoxy-oleanan-28,13β-olide Isolated from E. platycarpa and Its Protective Effect in Experimental Diabetes. Biol. Pharm. Bull. 2007, 30, 1503–1510. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Baez, E.G. Evaluation of antidiabetic, antioxidant and antiglycating activities of the Eysenhardtia polystachya. Pharmacogn. Mag. 2014, 10, 404–418. [Google Scholar] [CrossRef]
- Domínguez-Villegas, V.; Domínguez-Villegas, V.; García, M.L.; Calpena, A.; Clares-Naveros, B.; Garduño-Ramírez, M.L. Anti-Inflammatory, Antioxidant and Cytotoxicity Activities of Methanolic Extract and Prenylated Flavanones Isolated from Leaves of Eysehardtia platycarpa. Nat. Prod. Commun. 2013, 8, 177–180. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2015, 15, 237–250. [Google Scholar] [CrossRef]
- Andrade-Carrera, B.; Clares, B.; Noé, V.; Mallandrich, M.; Calpena, A.; García, M.L.; Garduño-Ramirez, M. Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells. Molecules 2017, 22, 1553. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Fábrega, M.-J.; Garduño-Ramírez, M.L.; Clares, B. Nanoemulsion strategy of pioglitazone for the treatment of skin inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.-M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; et al. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villegas, V.; Clares, B.; García-López, M.; Calpena-Campmany, A.C.; Bustos-Zagal, P.; Garduño-Ramirez, M. Development and characterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Salgado, P.; Andrade-Carrera, B.; Garduño-Ramírez, M.L.; Alvarado, H.L.; Calpena, A. Quantification of one Prenylated Flavanone from Eysenhardtia platycarpa and four derivatives in Ex Vivo Human Skin Permeation Samples Applying a Validated HPLC Method. Biomolecules 2020, 10, 889. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C.; Mira, L.; Corvo, M.L. Molecular Mechanisms of Anti-Inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Liang, L.-F.; Guo, Y.-W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012, 33, 1217–1245. [Google Scholar] [CrossRef]
- Rauh, L.K.; Horinouchi, C.D.; Loddi, A.M.; Pietrovski, E.F.; Neris, R.; Souza-Fonseca-Guimaraes, F.; Buchi, D.F.; Biavatti, M.W.; Otuki, M.F.; Cabrini, D.A. Effectiveness of Vernonia scorpioides Ethanolic extract against skin inflammatory processes. J. Ethnopharmacol. 2011, 138, 390–397. [Google Scholar] [CrossRef]
- Young, J.M.; Spires, D.A.; Bedord, C.J.; Wagner, B.; Ballaron, S.J.; De Young, L.M. The Mouse Ear Inflammatory Response to Topical Arachidonic Acid. J. Investig. Dermatol. 1984, 82, 367–371. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Regué, J.Q.; Garcia-Sala, X.; Montañés, A.B.; Lamuela-Raventos, R.M. In Vivo Anti-inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Sanaki, T.; Kasai-Yamamoto, E.; Yoshioka, T.; Sakai, S.; Yuyama, K.; Fujiwara, T.; Numata, Y.; Igarashi, Y. Direct Involvement of Arachidonic Acid in the Development of Ear Edema via TRPV3. J. Oleo Sci. 2017, 66, 591–599. [Google Scholar] [CrossRef] [PubMed][Green Version]

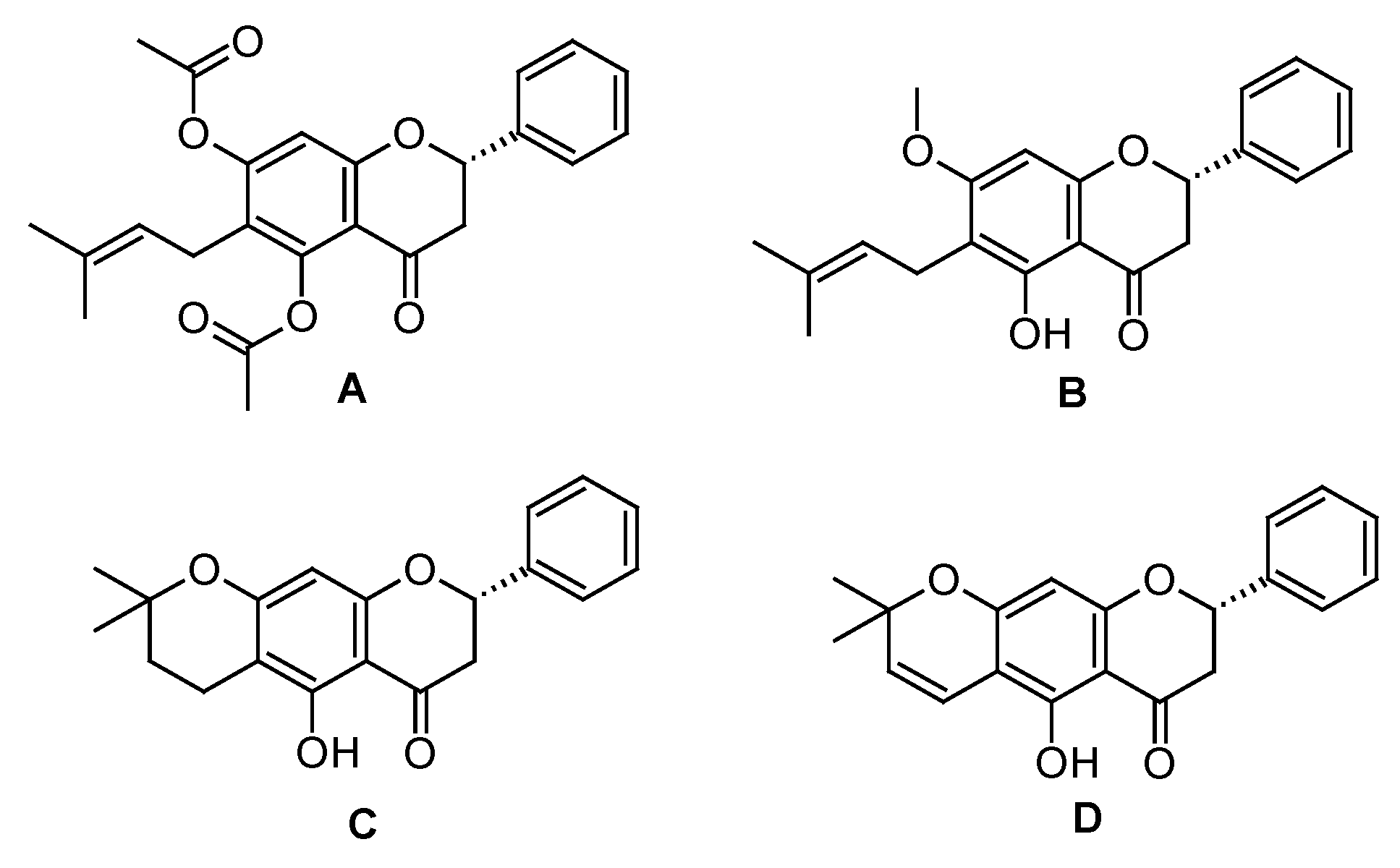
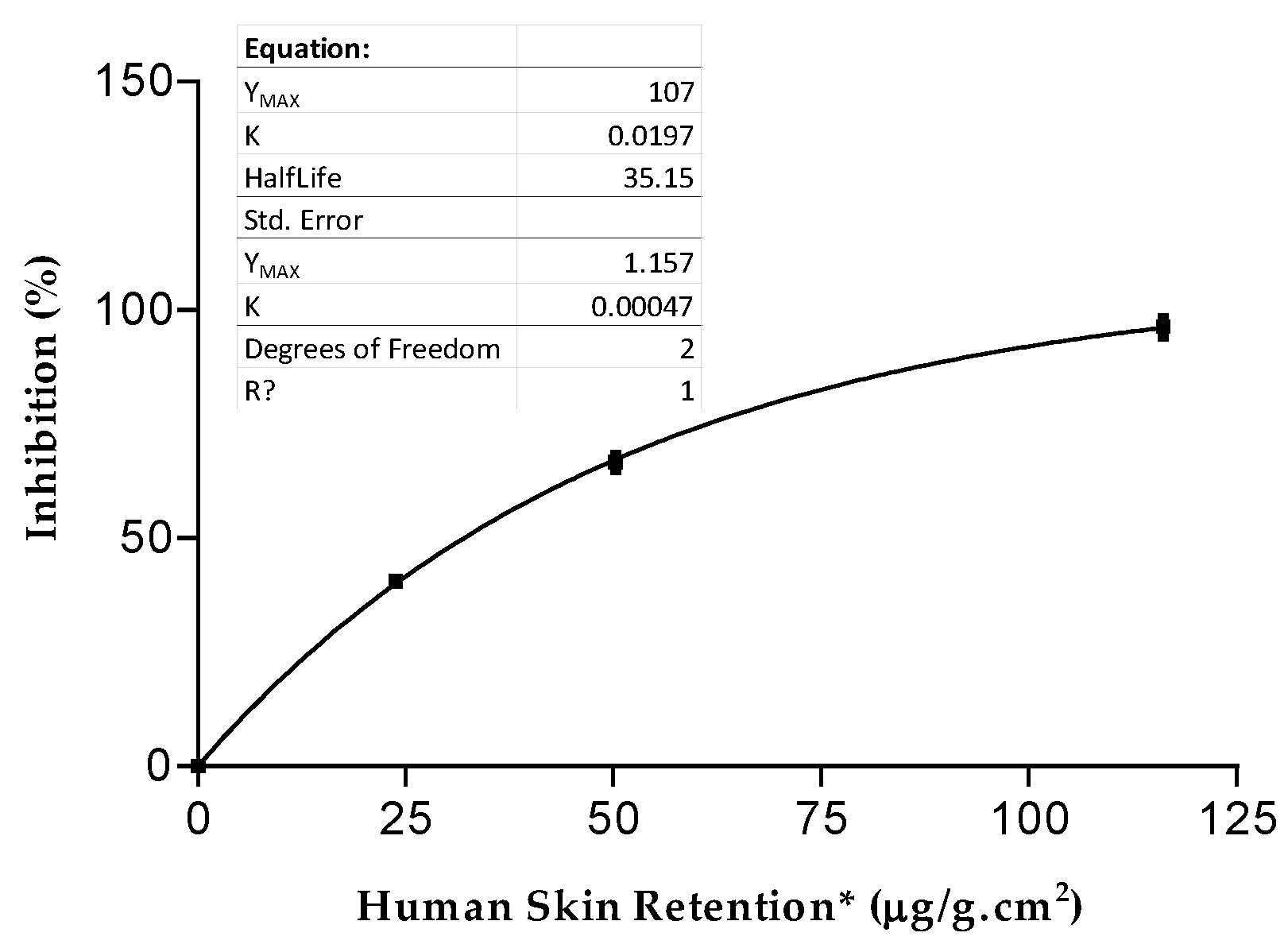
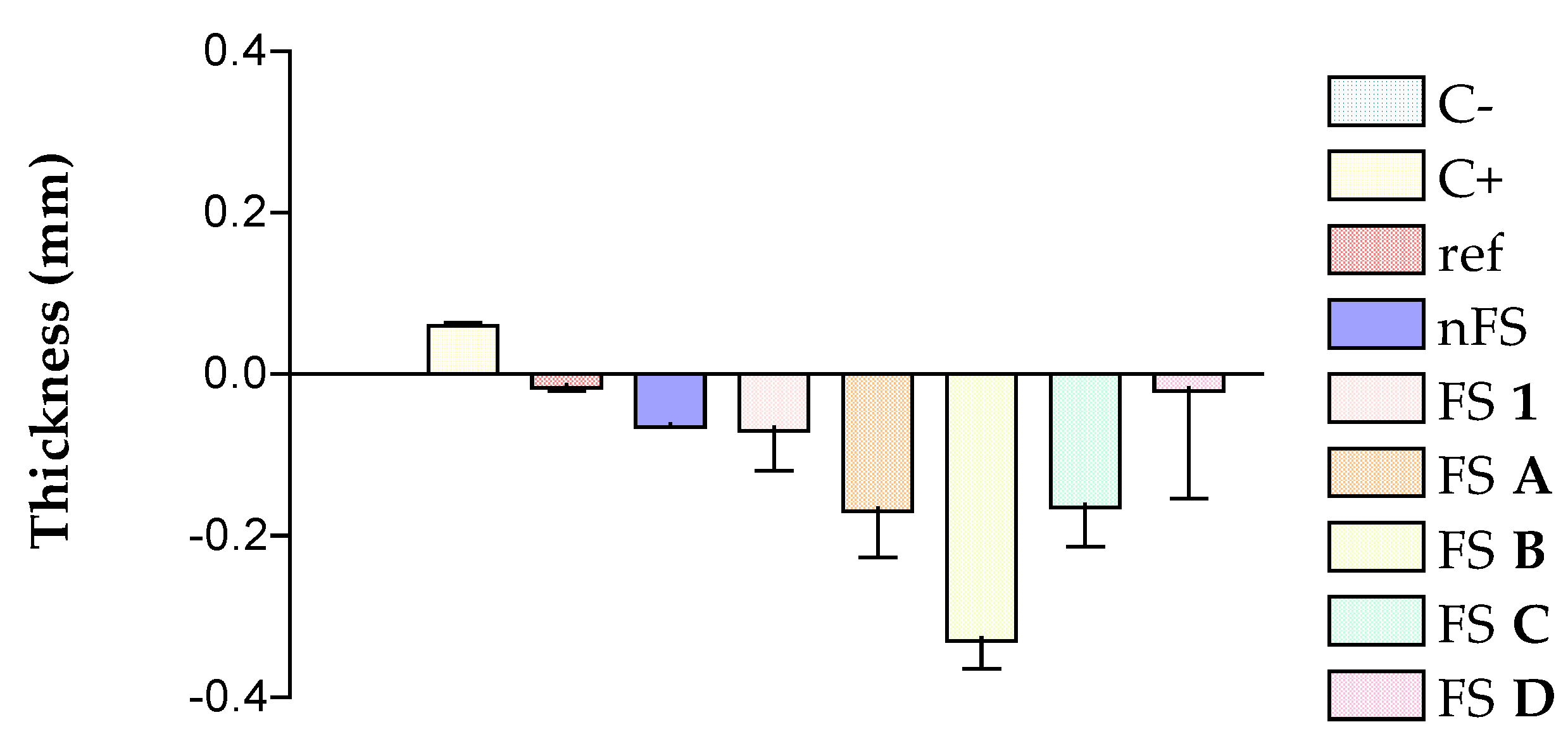
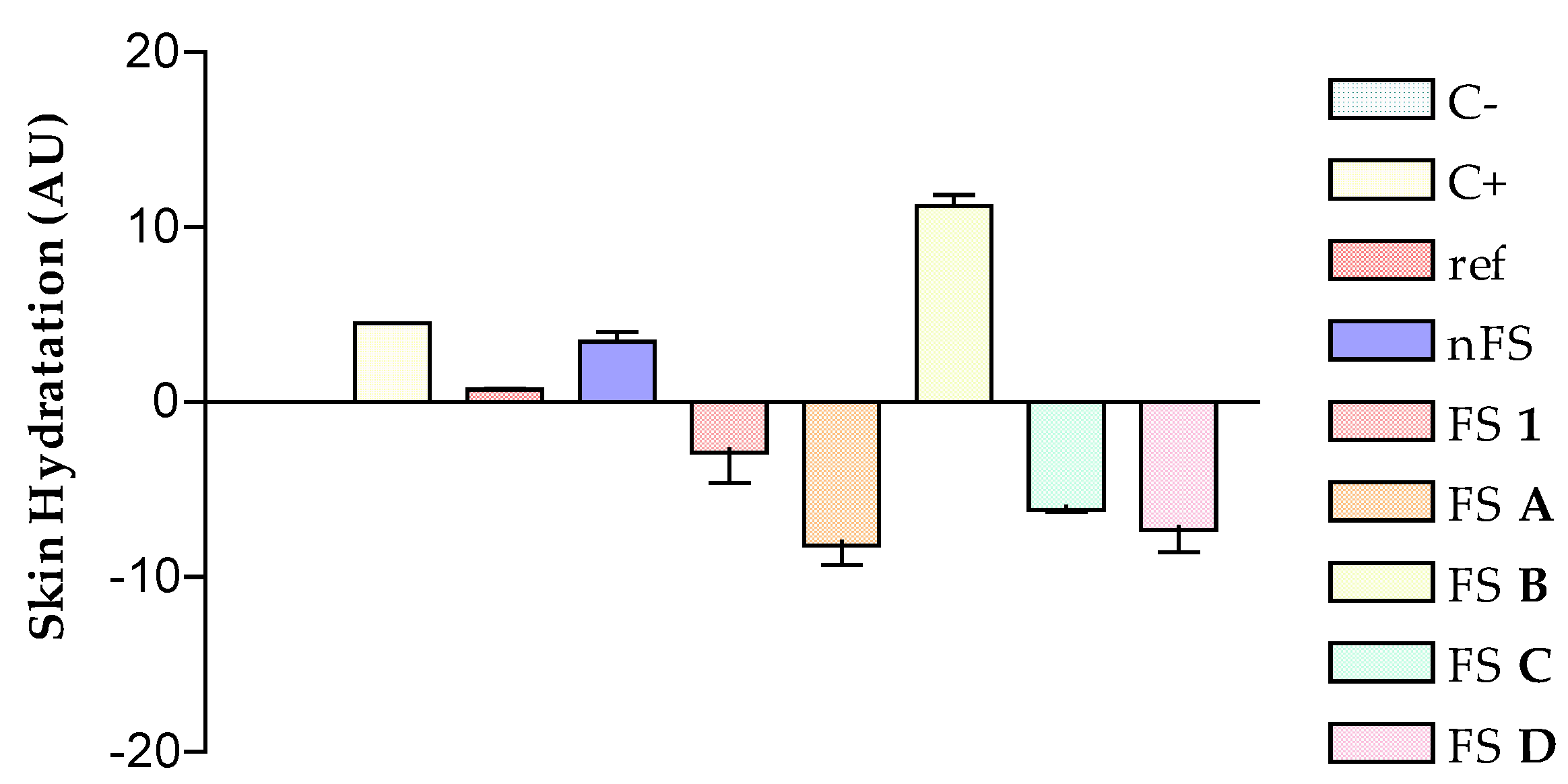
| Solutions | FS 1 | FS A | FS B | FS C | FS D | Indomethacin |
|---|---|---|---|---|---|---|
| % Inhibition | 66.67 ± 1.55 | 10.27 ± 0.21 | 25.69 ± 0.52 | 40.61 ± 0.81 | 96.27 ± 1.93 | 91.35 ± 0.47 |
| Human Skin Retention * (μg/g.cm2) | 50.22 ± 7.51 | 321.52 ± 45.23 | 381.75 ± 57.26 | 23.78 ± 5.46 | 116.14 ± 17.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos-Salgado, P.; Rodríguez-Lagunas, M.J.; Domínguez-Villegas, V.; Andrade-Carrera, B.; Calpena-Campmany, A.; Garduño-Ramírez, M.L. Ex Vivo and In Vivo Anti-inflammatory Evaluations of Modulated Flavanones Solutions. Proceedings 2021, 78, 23. https://doi.org/10.3390/IECP2020-08657
Bustos-Salgado P, Rodríguez-Lagunas MJ, Domínguez-Villegas V, Andrade-Carrera B, Calpena-Campmany A, Garduño-Ramírez ML. Ex Vivo and In Vivo Anti-inflammatory Evaluations of Modulated Flavanones Solutions. Proceedings. 2021; 78(1):23. https://doi.org/10.3390/IECP2020-08657
Chicago/Turabian StyleBustos-Salgado, Paola, María J. Rodríguez-Lagunas, Valeri Domínguez-Villegas, Berenice Andrade-Carrera, Ana Calpena-Campmany, and María Luisa Garduño-Ramírez. 2021. "Ex Vivo and In Vivo Anti-inflammatory Evaluations of Modulated Flavanones Solutions" Proceedings 78, no. 1: 23. https://doi.org/10.3390/IECP2020-08657
APA StyleBustos-Salgado, P., Rodríguez-Lagunas, M. J., Domínguez-Villegas, V., Andrade-Carrera, B., Calpena-Campmany, A., & Garduño-Ramírez, M. L. (2021). Ex Vivo and In Vivo Anti-inflammatory Evaluations of Modulated Flavanones Solutions. Proceedings, 78(1), 23. https://doi.org/10.3390/IECP2020-08657







