Published: 1 December 2020
1. Introduction
Drug solubility is a crucial element for a safe and efficacious formulation, as the absorption and bioavailability depend on its solubility. Furthermore, to display its efficacy, a drug molecule desires water solubility and permeability, which may be absorbed in the site of action. More than 90% of new chemical molecules have low water solubility [
1]. Several techniques can be used to boost the solubility, like chemical and physical techniques. Cyclodextrin is widely used to enhance drug solubility by altering the physicochemical properties of the drugs [
2]. Cyclodextrin forms the inclusion complex with guest molecules by fitting in their cavity, which satisfies the structural specifications and forces required to form a complex [
3,
4]. The inclusion complexes showed improved drug absorption, rapid drug release, and decreased side effects [
5]. The cyclodextrin-based inclusion complex with the lipophilic drug has risen to a high position in the pharmaceutical field, as it can alter the physicochemical properties of drugs [
6]. These amendments have an imperious part in drug delivery.
Iloperidone (ILO) is an atypical antipsychotic drug used to treat schizophrenia approved by USFDA in May 2009 [
7]. Schizophrenia is a chronic and severe mental disorder affecting more than 20 million individuals globally. ILO is a potent drug, as it shows better activity in a comparatively lower dose (12–16 mg) and acts as a dopaminergic and serotonergic antagonist. Tablets of ILO exist in several strengths, ranging from 1 mg to 12 mg. Moreover, it has less affection for the histamine and its receptor, the lowest sedation-related complications, and substantial progress in extrapyramidal symptoms [
8,
9,
10]. ILO is a Biopharmaceutical Classification Systems (BCS) class II drug with low solubility and high permeability.
This work aimed to prepare a complex with sulfobutyl ether-β-cyclodextrin (SEβCD) to improve solubility and dissolution, consequently enhancing bioavailability. The inclusion complex was characterized by saturation solubility, FTIR, differential scanning calorimetry (DSC), and multimedia dissolution.
2. Experiments
2.1. Materials
ILO and SEβCD were a kind gift from Symed Labs, Hyderabad, and Cyclolab. Purified water, analytical grade reagents, and chemicals were used to prepare the solution.
2.2. Method
2.2.1. Phase Solubility Studies
Higuchi and Connors defined the method used to perform the phase solubility studies for ILO with SEβCD. A UV spectrophotometer analyzed the samples at 229 nm. Complexation efficiency (CE) is the solubility of guest molecules by cyclodextrin (Equation (1)). The effect of cyclodextrin on solubility was analyzed from the solubility graph. The stability constant (Kc) was calculated from the ILO solubility graph in water (Equation (2)).
An equation:
where CE is complexation efficiency.
where K
c is stability constant and S
0 is the solubility of ILO only.
2.2.2. Formulation of the Solid Inclusion Complex
The kneading method was used to prepare the inclusion complex, which provides maximum solubility [
11]. The drug and SEβCD were calculated precisely in a ratio of 1:1. A uniform paste of SEβCD was formulated in the mortar by adding an adequate portion of methanol: water (1:1) followed by the addition of ILO, with continuous kneading for 45 min. The suitable paste consistency was maintained by adding a sufficient quantity of methanol: water (1:1). The complex was dried using a preheated hot air oven at 55 °C for 6 h. The dried complex was crushed, passed through sieve no. 44, and kept in a sealed container [
12].
2.3. Characterization of the Solid Inclusion Complex
2.3.1. Saturation Solubility
The solid inclusion complex’s saturation solubility was analyzed by adding an extra quantity of inclusion complex to achieve a steady state in a conical flask containing 10 mL phosphate buffer (pH 6.8), acetate buffer (pH 4.5), and 0.1 N HCl buffer. The conical flasks containing various solvents were kept in an orbital shaker for 24 h at 100 rpm at ambient temperature. After reaching the steady state, the sample was filtered through a 0.45 µm syringe filter and analyzed spectrophotometrically at 229 nm [
11].
2.3.2. Drug Content
The 10 mg equivalent solid inclusion complex was weighed accurately and dispersed in methanol. Subsequently, it was subjected to an orbital shaker for an hour to confirm the complete extraction of ILO. It was then filtered and estimated spectrophotometrically at 229 nm.
2.3.3. Attenuated Total Reflection-Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
The FTIR of ILO, SEβCD, and the inclusion complex was analyzed using the ATR-FTIR spectrometer (Perkin Elmer) at a broad range of 4000–400 cm−1. The sample was kept at the diamond substrate, and the pressure was applied with a compression bar. The software converts the ATR to absorbance. The characteristic peaks were analyzed compared with ILO and SEβCD to check the interaction between them.
2.3.4. Thermal Analysis
The thermal analysis of ILO, SEβCD, and the inclusion complex was studied using Mettler Toledo’s DSC. An empty aluminum plate was used as a reference. Around 5 mg of each test sample were pursed in an aluminum plate, and thermograms were found under a nitrogen gas flow of 10 mL/min. The thermal analysis was conducted at a heating rate of 10 °C/min from 30–300 °C.
2.3.5. Multimedia Dissolution Study
An in-vitro analysis of ILO and the inclusion complex (in capsule shell) was carried out using Dissolution apparatus USP Type II (Paddle type) (Electrolab, India). A phosphate buffer (pH 6.8), acetate buffer (pH 4.5), and 0.1 N HCl buffer (500 mL) were used as dissolution media at 37 ± 0.5 °C at 50 rpm. 10 mL aliquot of dissolution media was taken at pre-determined time intervals. The drug release profile of ILO and the inclusion complex were studied and compared to check the release of drug in multimedia.
3. Results and Discussion
3.1. Experimentals
3.1.1. Phase Solubility Studies
The phase solubility studies are a crucial parameter during the development of the inclusion complex with the drug. The drug’s (ILO) solubility curve in aqueous SEβCD was carried out, which showed that ILO’s solubility improved linearly, with an increase in the concentration of SEβCD. ILO showed an A
L-type graph, demonstrating the first-order dependency of the interaction with SEβCD. The linear A
L-type of the solubility curve suggests a 1:1 ratio for the complexation of ILO and SEβCD (
Figure 1,
Table 1). The higher value of CE and K
C specifies the stable formation of the complex by interacting with SEβCD. Kim et al. showed an enhanced intrinsic solubility, resulting in an improved CE in the presence of SEβCD by forming salt [
13].
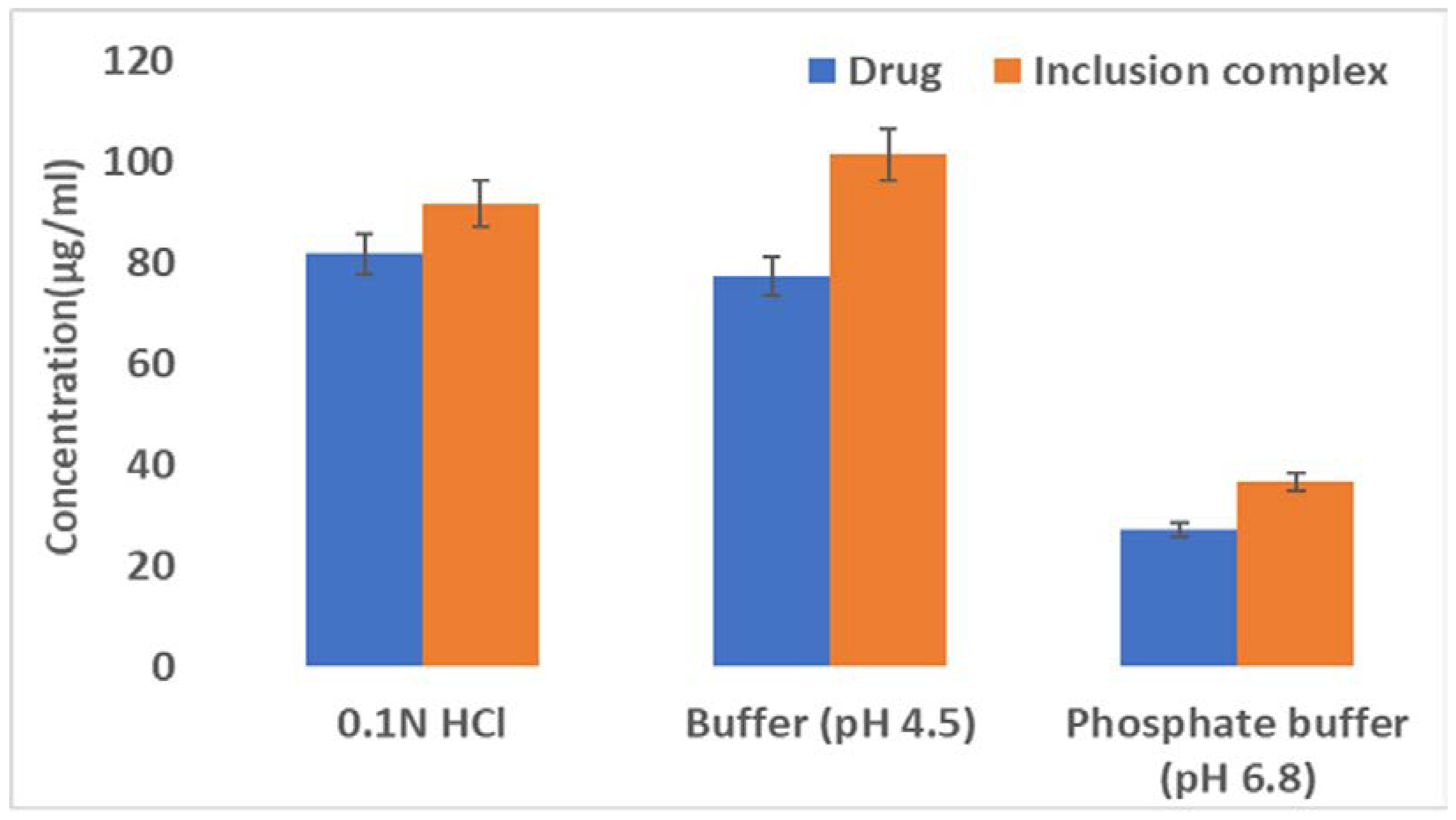
3.1.2. Saturation Solubility and Drug Content
The saturation solubility of ILO and the inclusion complex in a phosphate buffer (pH 6.8), acetate buffer (pH 4.5), and HCl buffer is represented in
Figure 2. The improved solubility of ILO in multimedia compared with plain ILO resulted from the inclusion complex of ILO and SEβCD. The percent drug content was found to be 99.23 ± 0.49, which complies with specifications.
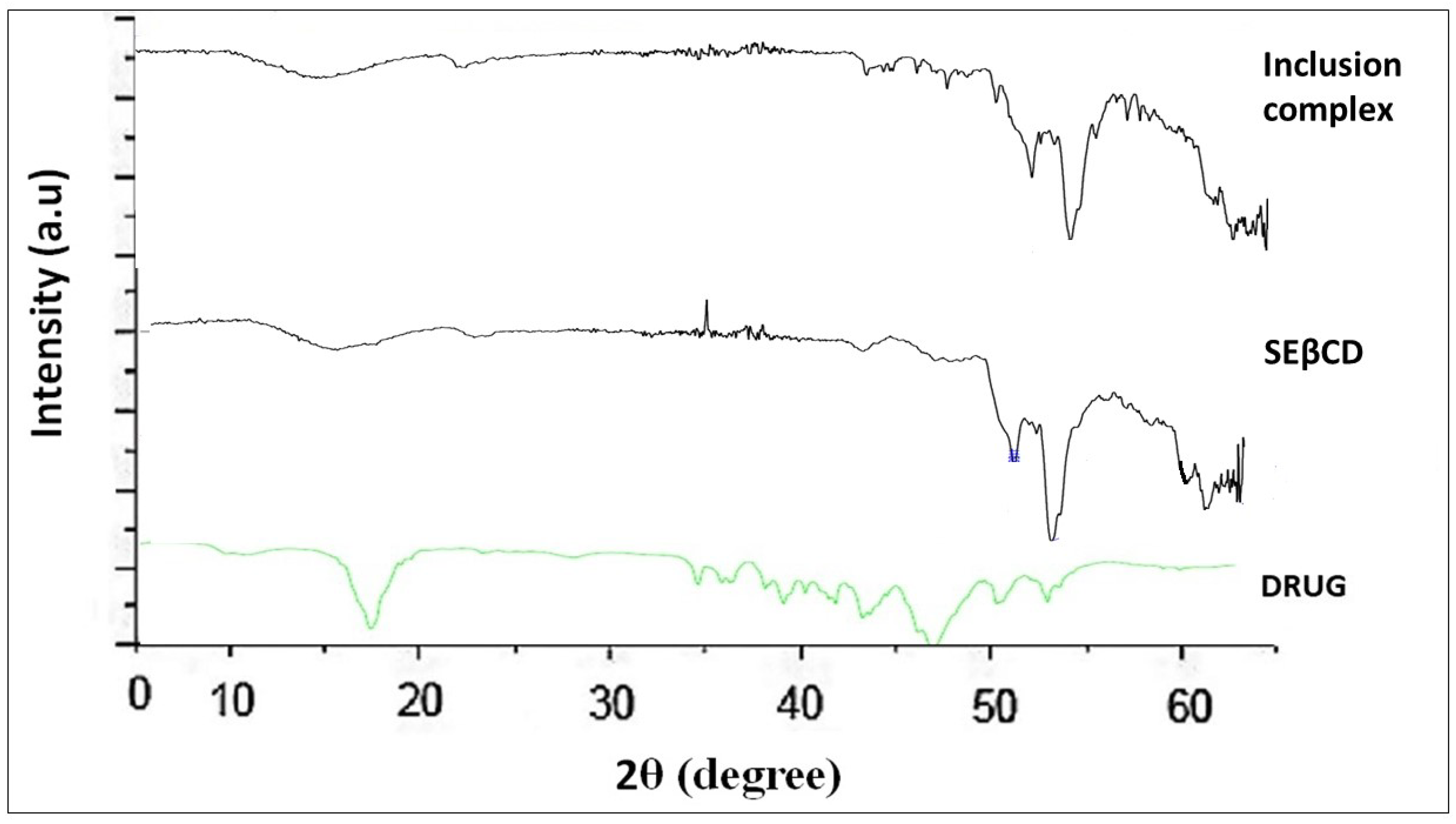
3.1.3. ATR-FTIR
ATR-FTIR is a convenient method to evaluate the drug (guest) and SEβCD (host) solid-state interaction. In IR spectra, detected peaks describe distinct functional groups that may change or modify the intensity of contacting host molecules by forming an inclusion complex, signifying effective complexation [
14]. The ATR-FTIR spectra displayed C-F stretch at 1262 c m
−1, N-O stretching at 1352 cm
−1, C=O stretching at 1668 cm
−1, and 2949 cm
−1 due to C-H stretching vibration. The spectrum of SEβCD is mainly characterized at ~1644 cm
−1 and reflects the H-O-H stretching of water molecules, whereas the peaks at ~1153 cm
−1 and ~1032 cm
−1 are attributed to C-H and C-O stretching vibrations. The IR spectra of the inclusion complex depicted a decrease in intensity, the alteration, and the disappearance of some distinctive IR bands of ILO. The interaction of ILO with SEβCD was confirmed by a significant shift of some distinctive bands of ILO (
Figure 3).
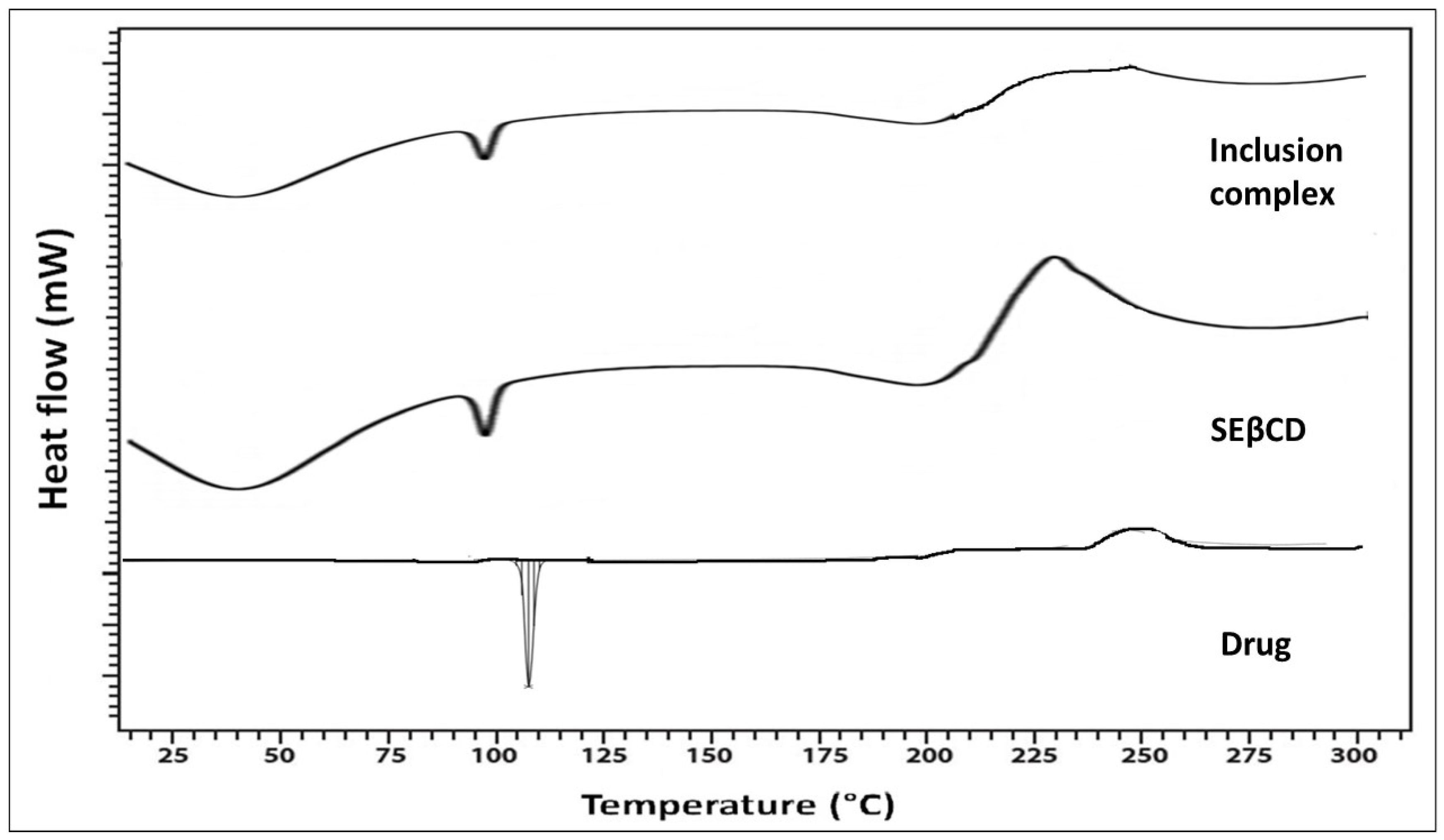
3.1.4. Thermal Analysis
Thermal analysis delivers additional suggestions for forming the inclusion complex due to the melting or decay of drugs that may shift to different temperatures or disappear when formulated with SEβCD [
15]. The thermogram of ILO, SEβCD, and the inclusion complex is shown in
Figure 4. The DSC of ILO displayed a sharp and well-defined endothermic peak at 120 °C, conforming to the drug’s (ILO) melt point. In the endotherm of SEβCD, a broad peak was detected at 86 °C. The endotherm of ILO utterly disappeared into the stable inclusion complex. The amorphization of the drug describes an enhancement in the dissolution profile of ILO.
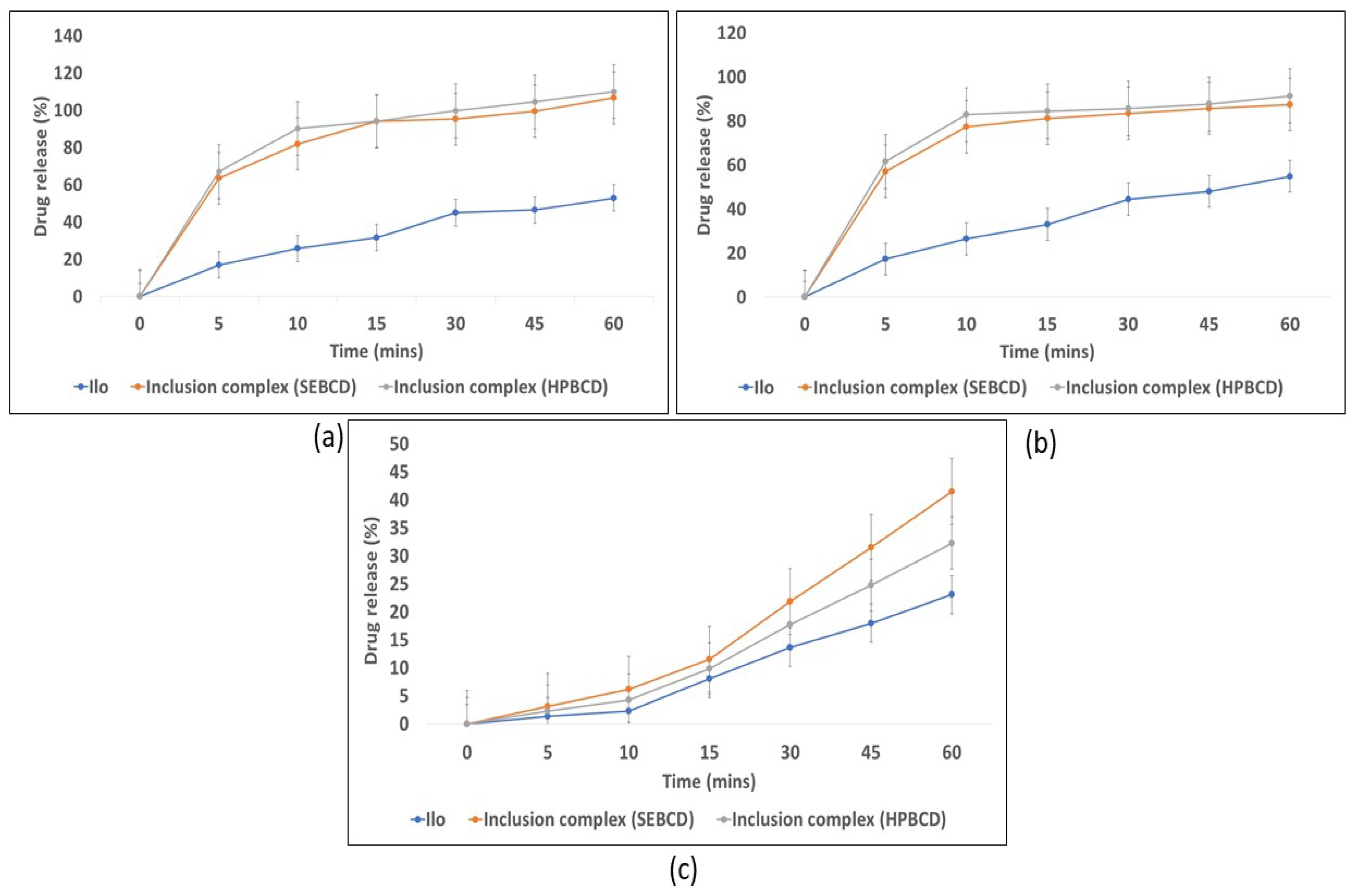
3.1.5. Multimedia Dissolution Study
The dissolution study was analyzed to examine the enhanced solubility of ILO from the inclusion complex. The multimedia dissolution of the inclusion complex was done to identify the behavior of the complex over a wide range of pH. The dissolution of the inclusion complex and ILO was done in a 0.1 N HCl buffer, acetate buffer (pH 4.5), and phosphate buffer (pH 6.8), shown in
Figure 5c respectively. More than 80% of the drug release was found in 0.1 N HCl
Figure 5a. and buffered pH 4.5
Figure 5b, which depicts the significant difference between the drug (
p ≤ 0.05). In phosphate buffer (pH 6.8), no significant difference was observed between the drug
Figure 5c. In multimedia, the inclusion complex showed no significant difference when compared with the HPβCD-based inclusion complex [
16]. The multimedia dissolution data show that as the buffer’s pH decreased from 6.8 to 4.5 and 1.2, the dissolution rate was significantly enhanced.
4. Conclusions
In this research article, we evaluated the inclusion of SEβCD to enhance the solubility of the drug. Thermal analysis (DSC) and FTIR depict the reduction in crystallinity and increase the host-guest interaction, suggesting the inclusion of complex formation. SEβCD displayed improved complexation efficacy and solubility and more stability, as detected from the FTIR study. The inclusion complex might be attributed to the enhanced dissolution of the drug. Thus, it can be concluded that SEβCD-based inclusion complexes can lead to an increase in solubility, which may enhance the drug’s bioavailability.
Author Contributions
V.L. conceived and designed the experiments; R.B. performed the experiments; V.L. and R.B. analyzed the data; SERB, Cyclolab, Symed lab, and SPP SPTM contributed reagents/materials/analysis tools; R.B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Exclude this statement.
Acknowledgments
This research was supported by the Science and Engineering Research Board, File Number: CRG/2018/003176. The authors would like to acknowledge the help provided by Symed lab and Cyclolab in the form of gift samples.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ILO | Iloperidone |
| SeβCD | sulfobutyl ether-β-cyclodextrin |
| BCS | Biopharmaceutical Classification Systems |
| ATR-FTIR | Attenuated total reflection- Fourier-transform infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| CE | Complexation efficiency |
| Kc | stability constant |
References
- Gribbon, P.; Andreas, S. High-throughput drug discovery: What can we expect from HTS? Drug Discov. Today 2005, 1, 17–22. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Danel, C.; Azaroual, N.; Chavaria, C.; Odou, P.; Martel, B.; Vaccher, C. Comparative study of the complex forming ability and enantioselectivity of cyclodextrin polymers by CE and 1H NMR. Carbohydr. Polym. 2013, 92, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, M.V.G.; Vieira, J.V.F.; Da Silva, C.W.; Barison, A.; Andrade, G.R.S.; Da Costa, N.B.; Barboza, F.M.; Nadal, J.M.; Novatski, A.; Farago, P.V.; et al. Host-guest complexes of 2-hydroxypropyl-β-cyclodextrin/β-cyclodextrin and nifedipine: 1 H NMR, molecular modeling, and dissolution studies. J. Mol. Struct. 2017, 1150, 146–154. [Google Scholar] [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J. Cyclodextrins as Drug Carriers in Pharmaceutical Technology: The State of the Art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Park, J.-S. Solubility Enhancers for Oral Drug Delivery. Am. J. Drug Deliv. 2004, 2, 113–130. [Google Scholar] [CrossRef]
- iloperidone—U.S. Food and Drug Administration Search Results [Internet]. Search.usa.gov. 2020. Available online: https://search.usa.gov/search?query=iloperidone&affiliate=fda1 (accessed on 20 May 2020).
- Weiden, P.J. Iloperidone for the Treatment of Schizophrenia: An Updated Clinical Review. Clin. Schizophr. Relat. Psychoses 2012, 6, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Berg, P.H. The psychosis of schizophrenia: Prevalence, response to atypical antipsychotics, and prediction of outcome. Biol. Psychiatry 1999, 46, 361–364. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Illum, L.; Davis, S.S. Schizophrenia and Drug Delivery Systems. J. Drug Target. 2000, 8, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Londhe, V.Y.; Shirsat, R. Formulation and Characterization of Fast-Dissolving Sublingual Film of Iloperidone Using Box–Behnken Design for Enhancement of Oral Bioavailability. AAPS PharmSciTech 2018, 19, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Soniwala, M.M.; Patel, P.R.; Ansuri, M.S.; Parikh, R.K.; Goel, M.C. Various approaches in dissolution enhancement of Rofecoxib. Indian J. Pharm. Sci. 2005, 67, 61–63. [Google Scholar]
- Kim, Y.; Oksanen, A.D.; Massefski, W., Jr.; Blake, F.J.; Duffy, M.E.; Chrunyk, B. Inclusion complexation of ziprasidone mesylate with b-cyclodextrin sulfobutyl ether. J. Pharm. Sci. 1998, 87, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Hirlekar, R. Multicomponent cyclodextrin system for improvement of solubility and dissolution rate of poorly water soluble drug. Asian J. Pharm. Sci. 2019, 14, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Li, X.; Zheng, Y. Inclusion complex of nateglinide with sulfobutyl ether b-cyclodextrin: Preparation, characterization and water solubility. J. Mol. Struct. 2017, 1141, 328–334. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Pawar, A.; Kundaikar, H. Studies on spectral characterization and solubility of hydroxypropyl β-cyclodextrin/iloperidone binary and ternary complexes using different auxiliary agents. J. Mol. Struct. 2020, 1220, 128615. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).