Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice †
Abstract
:1. Introduction
2. Results
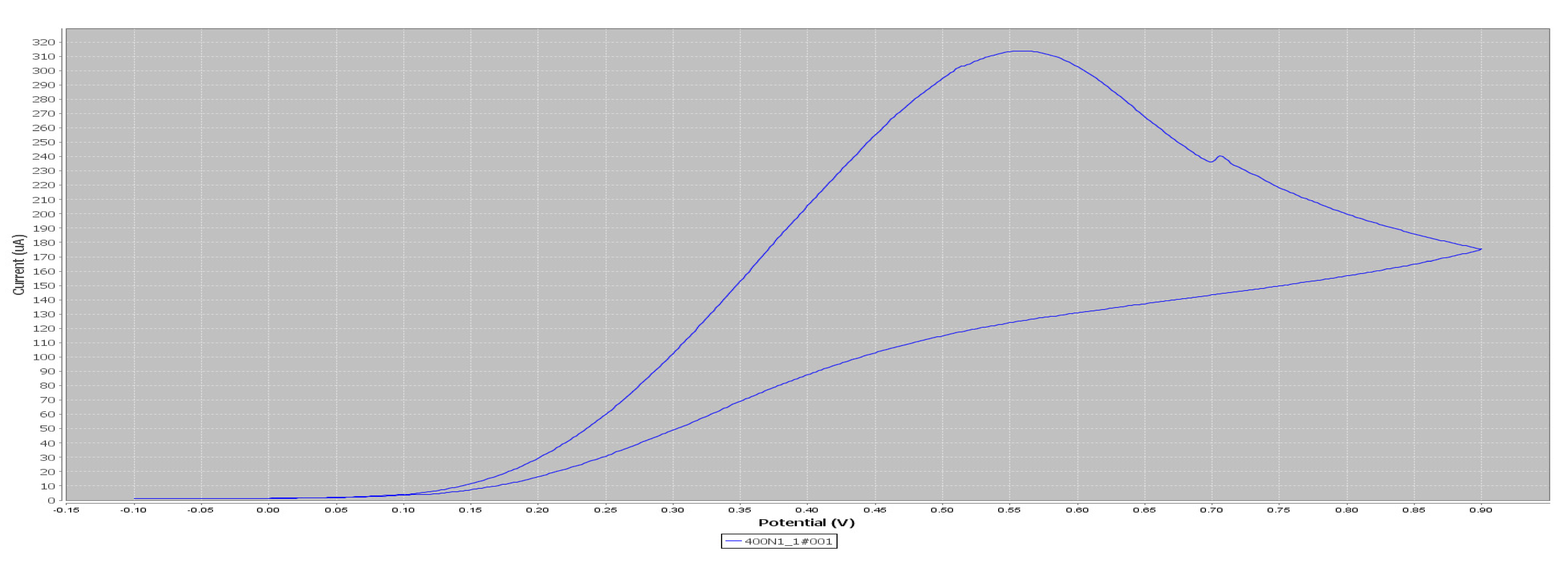
2.1. AA Content in Orange Juices
2.2. Ascorbic Acid Degradation during Storage
3. Discussions
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Q.; Peng, Y.; Zhu, C.; Pan, S. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara Cara. Food Chem. 2018, 265, 39–48. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; Fernández-Arias, M.; López-Hernández, J. A screening method for the determination of ascorbic acid in fruit juices and soft drinks. Food Chem. 2009, 116, 509–512. [Google Scholar] [CrossRef]
- Uchlyama, S.; Umetsu, Y. Concentration-step amperometric sensor of L-ascorbic acid using cucumber juice. Anal. Chim. Acta 1991, 255, 53–57. [Google Scholar] [CrossRef]
- Aguilar, K.; Garvín, A.; Ibarz, A.; Augusto, P.E.D. Ascorbic acid stability in fruit juices during thermosonication. Ultrason. Sonochemistry 2017, 37, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Guanghan, L.; Yu, W.; Leiming, Y.; Shuanglong, H. Determination of ascorbic acid in fruits and vegetables by stripping voltammetry on a glassy carbon electrode. Food Chem. 1994, 51, 237–239. [Google Scholar]
- Florou, A.B.; Prodromidis, M.I.; Tzouwara-Karayanni, S.M.; Karayannis, M.I. Fabrication and voltammetric study of lanthanum 2,6-dichlorophenolindophenol chemically modified screen printed electrodes. Application for the determination of ascorbic acid. Anal. Chim. Acta 2000, 423, 107–114. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S.; Wichitpanya, S.; Daengduang, S.; Puttota, S. Electrochemical sensor based on PANI/MnO2-Sb2O3 nanocomposite for selective simultaneous voltammetric determination of ascorbic acid and acetylsalicylic acid. J. Electroanal. Chem. 2016, 782, 192–201. [Google Scholar] [CrossRef]
- Silva, A.C.; Lourenço, A.S.; Ugulino de Araujo, M.C. Simultaneous voltammetric determination of four organic acids in fruit juices using multiway calibration. Food Chem. 2018, 266, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.O.; Ng, S.C.; Seow, S.H. Polybithiophene-modified electrode: Spectrophotometric detection of ascorbic acid. Synth. Met. 1994, 66, 177–183. [Google Scholar] [CrossRef]
- Yebra, M.C.; Cespón, R.M.; Moreno-Cid, A. Automatic determination of ascorbic acid by flame atomic absorption spectrometry. Anal. Chim. Acta 2001, 448, 157–164. [Google Scholar] [CrossRef]
- Zuo, R.; Zhou, S.; Zuo, Y.; Deng, Y. Determination of creatinine, uric and ascorbic acid in bovine milk and orange juice by hydrophilic interaction HPLC. Food Chem. 2015, 182, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Al Mughairy, B.; Al-Lawati, H.A.J.; Eldin, F. Characterization and application of nanocolloidal Mn(IV) in a chemiluminescence system for estimating the total phenolic content in pomegranate juices using a nanodroplet microfluidics platform Suliman. Sens. Actuators B Chem. 2018, 277, 517–525. [Google Scholar] [CrossRef]
- Falkova, M.T.; Bulatov, A.V.; Pushina, M.O.; Ekimov, A.A.; Alekseeva, G.M.; Moskvin, L.N. Multicommutated step wise injection determination of ascorbic acid in medicinal plants and food samples by capillary zone electrophoresis ultraviolet detection. Talanta 2015, 133, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Sha, R.; Vishnu, N.; Kumar, R.; Badhulika, S. Disposable, efficient and highly selective electrochemical sensor based on Cadmium oxide nanoparticles decorated screen-printed carbon electrode for ascorbic acid determination in fruit juices. Nano Struct. Nano Objects 2018, 16, 96–103. [Google Scholar] [CrossRef]
- Kuss, S.; Compton, R.G. Electrocatalytic detection of ascorbic acid using N,N,N’,N’-tetramethyl-para-phenylene-diamine (TMPD) mediated oxidation at unmodified gold electrodes; reaction mechanism and analytical application. Electrochim. Acta 2017, 242, 19–24. [Google Scholar] [CrossRef]
- Grudić, V.V.; Blagojević Vesna, Z.; Vukašinović-Pešić, L.; Brašanac, S.R. Kinetics of degradation of ascorbic acid by cyclic voltammetry method. Chem. Ind. Chem. Eng. Q. 2015, 21, 351–357. [Google Scholar] [CrossRef]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- Remini, H.; Mertz, C.; Belbahi, A.; Achir, N.; Dornier, M.; Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Dobrinas, S.; Radu, G.L. Spectrophotometric determination of ascorbic acid in grapes with the Prussian Blue reaction. Ovidius Univ. Ann. Chem. 2012, 23, 174–179. [Google Scholar] [CrossRef]

| Sample | The Content of Ascorbic Acid mg AA/100 mL | |
|---|---|---|
| Spectrometric Method | Voltammetric Method | |
| 1 | 29.98 ± 0.24 | 30.13 ± 0.23 |
| 2 | 32.08 ± 0.31 | 32.13 ± 0.37 |
| 3 | 42.14 ± 0.43 | 42.27 ± 0.35 |
| 4 | 38.22 ± 0.22 | 38.28 ± 0.44 |
| Sample | Temperature | Zero-Order Kinetic Model | First-Order Kinetic Model | ||
|---|---|---|---|---|---|
| Rate Equation | R2 | Rate Equation | R2 | ||
| 1 | 277 K | y = −0.4406x + 30.605 | 0.9979 | y = −0.0164x + 0.0062 | 0.9998 |
| 2 | y = −0.3797x + 32.607 | 0.9919 | y = −0.0132x + 0.007 | 0.9998 | |
| 3 | y = −0.3734x + 42.692 | 0.9979 | y = −0.0094x + 0.0027 | 0.9999 | |
| 4 | y= −0.3374x + 38.599 | 0.9995 | y= −0.0089x + 0.0007 | 0.9997 | |
| 1 | 295 K | y= −0.553x + 30.657 | 0.9992 | y = −0.0006x − 0.0011 | 0.9998 |
| 2 | y= −0.52x + 32.674 | 0.9994 | y = −0.0006x + 0.0027 | 0.9998 | |
| 3 | y= −0.537x + 42.815 | 0.9998 | y = −0.0004 + 0.0009 | 0.9999 | |
| 4 | y= −0.525x + 38.829 | 0.9989 | y= −0.0005x + 0.0021 | 0.9993 | |
| Sample | Temperature | Rate Constant k (min−1) | t1/2 (min) | Temperature | Rate Constant k (min−1) | t1/2 (min) |
|---|---|---|---|---|---|---|
| 1 | 277 K | 5 × 10−4 | 1386 | 295 K | 6 × 10−4 | 1155 |
| 2 | 4 × 10−4 | 1732.5 | 5 × 10−4 | 1386 | ||
| 3 | 3 ×10−4 | 2310 | 4 × 10−4 | 1732.5 | ||
| 4 | 2.97 × 10−4 | 2333 | 4.5 × 10−4 | 1540 |
| Sample | E kJmol−1 | ΔH# Jmol−1 | ΔS# J·mol−1K−1 | ΔG# kJmol−1 | |
|---|---|---|---|---|---|
| 277K | 295 K | ||||
| 1 | 7289.24 | −992.6 | −308.48 | 84,456.36 | 90,009 |
| 2 | 8422.80 | −1331.9 | −310.67 | 84,723.69 | 90,315.75 |
| 3 | 10,856.64 | −1868.1 | −313.60 | 84,999.1 | 90,643.9 |
| 4 | 15,689.54 | −2930.6 | −314.75 | 84,,255.15 | 89,920.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soceanu, A.; Matei, N.; Dobrinas, S.; Popescu, V. Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice. Proceedings 2021, 70, 55. https://doi.org/10.3390/foods_2020-07693
Soceanu A, Matei N, Dobrinas S, Popescu V. Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice. Proceedings. 2021; 70(1):55. https://doi.org/10.3390/foods_2020-07693
Chicago/Turabian StyleSoceanu, Alina, Nicoleta Matei, Simona Dobrinas, and Viorica Popescu. 2021. "Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice" Proceedings 70, no. 1: 55. https://doi.org/10.3390/foods_2020-07693
APA StyleSoceanu, A., Matei, N., Dobrinas, S., & Popescu, V. (2021). Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice. Proceedings, 70(1), 55. https://doi.org/10.3390/foods_2020-07693





