Abstract
The objective of this study was to evaluate the nutritional composition, techno-functional, and in vitro physiological properties of flours from six different insect species (mealworm, beetle, caterpillar, ant, locust, and cricket). The chemical composition of insects was evaluated following the standard methods (AOAC). Bulk density, water holding capacity, oil holding capacity, water absorption capacity, swelling capacity, emulsifying activity, foaming capacity, and gelation capacity were measured. In vitro antioxidant capacity was measured by the direct ABTS method. Hypoglycemic (glucose adsorption and the inhibition of α-amylase, glucose diffusion, and starch hydrolysis) and hypolipidemic (cholesterol and bile salts binding and lipase inhibition capacities) were investigated using in vitro methods. Insect flours exhibited a high content of protein (39.4%–58.1%) and fat (17.7%–50.1%) as main components, although the presence of chitin in ant samples was also highlighted. The techno-functional properties showed high oil holding, swelling, and emulsifying capacities in all insect flours analyzed, besides bulk density, hydration properties, and foaming capacity showing average values and no gelation capacity. Insects showed high antioxidant capacity (179–221 mg Trolox equivalents/g). Moreover, these edible insect flours revealed effective hyperglycemic and hyperlipidemic properties. Insect flours inhibited α-amylase activity (47.1%–98.0%) and retarded glucose diffusion (17.2%–29.6%) and starch hydrolysis (18.2%–88.1%). Likewise, they bound cholesterol and bile salts (8.4%–98.6%) and inhibited lipase activity (8.9%–47.1%). Hence, these insect flours might be of great interest to the food industry, being a healthy source of protein, exerting a positive impact on functional food properties, and potentially preventing the development of diseases associated with hyperglycemia and hyperlipidemia.
Keywords:
insects; nutrients; sustainable food; protein; lipids; chitin; physicochemical properties; hypoglycemic; hypolipidemic 1. Introduction
The demand for more sustainable food production has generated attention in insect consumption. The main reason for this raised interest in insects is their nutritional characteristics: insects are a source of high-quality and easily digestible protein, fat, chitin, and a great variety of micronutrients. For these reasons, insects are presented as a feasible solution to the global food shortage due to overpopulation, estimated for 2050 [1]. Despite numerous benefits of edible insect consumption, consumer acceptance still remains one of the barriers to their utilization as a protein food source, especially in the developed countries where insects are viewed in disgust by the majority of the population [2]. On the contrary, there is a lack of scientifically based knowledge of insect processing to ensure food safety, primarily when these processes are carried out on an industrial scale [3]. Scientific evidence shows that edible insects’ nutritional quality is equivalent to and sometimes exceeds that of animal-based foods [4]. Besides, insects protein fraction could have biological activity by releasing antioxidant peptides after enzymatic hydrolysis [5]. However, little is known about edible insects’ beneficial physiological properties and other components’ potential role in these properties. Hence, insects could be a source of new ingredients with beneficial health properties. Thereupon, this work aimed to assess the nutritional composition, techno-functional, and in vitro physiological properties of flours from six different insect species (mealworm, beetle, caterpillar, ant, locust, and cricket).
2. Material and Methods
2.1. Materials
Six species of insects were purchased dried from different online stores specialized in the sale of insects for human consumption: Insectum (Valencia, Spain), BCN Insects (Barcelona, Spain), Europe Enthomophagie (Ruan, France), and Next Food (Ras al-Khaimah, United Arab Emirates). The analyzed edible insects corresponded to the four orders more consumed worldwide: mealworm (Tenebrio molitor, Coleoptera), beetle (Phyllophaga rugosa, Coleoptera), caterpillar (Nudaurelia melanops, Lepidoptera), ant (Oecophylla smaradigna, Hymenoptera), locust (Locusta migratoria, Orthoptera), and cricket (Acheta domesticus, Orthoptera). Samples were dehydrated by lyophilization, ground, and sieved, obtaining flours with a particle size of 0.5 mm.
2.2. Chemical Composition Analysis
2.2.1. Proximate Analysis
Crude protein, crude fat, and total ash were assessed following official AOAC procedures [6]. The nitrogen of chitin must be considered for the quantification of protein [7]. The protein content (%) was calculated as followed:
2.2.2. Determination of Chitin
Samples (1.6 g) were hydrolyzed with 1 M HCl at 100 °C for 30 min. Four sample repetitions were made (two samples for chitin determination and two samples for nitrogen in chitin determination) [7]. Two filtered hydrolysates of each sample were dried at 105 °C for 24 h. The other two hydrolysates were used for the nitrogen contained in chitin determination by the Kjeldahl method [6]. The chitin content (%) was calculated using the equation:
2.2.3. Determination of Trypsin Inhibitor
Insect flours were subjected to overnight extraction using 0.02 M sodium phosphate buffer pH 7.0 (1:10 w/v) at 4 °C and centrifuged (5000 × g, 25 min). The supernatants were stored at −20 °C. The protease-inhibitors concentration was analyzed using Nα-benzoyl-DL-arginine-p-nitroanilide hydrochloride (BAPNA) as a substrate [8].
2.3. In Vitro Antioxidant Capacity Determination
The direct in vitro antioxidant capacity was estimated by ABTS+ assay [9]. Insect flours (10 mg) were mixed with an ABTS+ solution (1.7 mL) for 5 min at 37 °C. All samples and standards were centrifuged (15,600 × g, 5 min), and the absorbance of the supernatants was measured at 734 nm. The results were expressed as mg Trolox equivalents per gram (mg TE/g) of the dry sample.
2.4. Physicochemical and Techno-Functional Properties
Bulk density (BD), water holding capacity (WHC), oil holding capacity (OHC), water absorption capacity (WAC), swelling capacity (SWC), emulsifying activity (EA), foaming capacity (FC), and gelation capacity (LGC, last gelation concentration), were measured according to published protocols [10].
2.5. Evaluation of the In Vitro Hypoglycaemic Properties
2.5.1. Glucose Adsorption Capacity
Edible insect flours (0.25 g) and 25 mL of glucose solution (10, 50, 100, 200 mM) were mixed [10]. They were incubated at 37 °C for 6 h and were centrifuged (3500× g, 15 min). The glucose assay kit (Megazyme KGLUC, Wicklow, Ireland) was used to determine the glucose adsorbed by the sample.
2.5.2. In Vitro α-Amylase Inhibition
Edible insect flours (0.25 g) were mixed with 1 mg of α-amylase (Sigma-Aldrich, Saint Louis, MO, USA) and a solution of potato starch (4% p/v, 10 mL) and incubated at 37 °C for 60 min. The samples were centrifuged (3500× g, 15 min) and the supernatant’s glucose content was determined using the assay kit KGLUC (Megazyme, Wicklow, Ireland). Residual amylase activity was determined in comparison with the control without insect flours [10].
2.5.3. In Vitro Glucose Dialysis Retardation Capacity
In vitro glucose dialysis retardation capacity was estimated by evaluating the glucose dialysis retardation index (GDRI) [10]. The solution was made with 0.5 g of edible insect flours and 25 mL of glucose solution (50 mM) and was dialyzed against distilled water (80 mL) at 37 °C for 0–150 min. Finally, the content of glucose in the dialysate was measured using the assay kit KGLUC (Megazyme, Wicklow, Ireland). The GDRI was calculated as follows:
2.5.4. In Vitro Starch Digestibility Retardation
As previously described [10], a solution was formulated adding 0.2 g of flours, 4 mg of α-amylase (Sigma Aldrich, MO, USA), and 10 mL of potato starch solution (4% p/v) dialyzed against distilled water (80 mL) at 37 °C for 0–150 min. The glucose assay kit was used to evaluate the glucose content in the dialysate.
2.6. Evaluation of the In Vitro Hypolipidemic Properties
2.6.1. Cholesterol Binding Capacity
Fresh egg yolks were diluted (nine times) using Milli-Q water and emulsified [10]. Edible insect flours (1 g) were mixed with diluted egg yolk (25 mL), and the pH was adjusted to 2.0 and 7.0. The flasks were incubated (37 °C, 2 h) with oscillation and centrifuged at 4000 × g for 15 min. The supernatant was diluted with acetic acid (90% v/v), mixed with o-phthalaldehyde and 96% H2SO4, and incubated under continuous stirring (60 °C, 30 min). The absorbance was measured at 550 nm. Pure cholesterol was used as a standard.
2.6.2. Sodium Cholate Binding Capacity
Edible insect flours (0.25 g) were combined with 50 mg of sodium cholate (Sigma-Aldrich, MO, USA) and 25 mL of NaCl solution (0.15 M, pH 7.0) [10]. The final solution was held on the shaker for 1 or 3 h, all of them at 37 °C and centrifuged at 4000 × g for 20 min. The absorption capacity was calculated as the percentage of absorbed sodium cholate. Samples were mixed with H2SO4 (45% v/v) and of furfural (0.3% v/v) and incubated at 65 °C for 30 min. Absorbance was measured at 620 nm. Sodium cholate was used as a standard.
2.6.3. In Vitro Pancreatic Lipase Inhibition
A mixture solution was formulated, adding 0.1 g of edible insect flour, 10 mL of sodium phosphate buffer (0.1 M, pH 7.2), 2 mL of olive oil, and 2 mL of pancreatic lipase solution (0.75 mg pancreatic lipase/mL phosphate buffer). Samples were incubated at 37 °C for 1 h [10]. The content of free fatty acid released was determined by titrating with NaOH (0.05 M). A similar method was carried out by adding bile salts (156.5 mg/mL) to evaluate the bile salts-binding effect on lipase activity. Lipase inhibitory activity (%) was defined as the percentage of decrease in the free fatty acid production rate over the control.
2.7. Statistical Analysis
Each sample was analyzed in triplicate (n = 3). Data were reported as mean ± standard deviation (SD). The data were analyzed using the t-test or one-way analysis of variance (ANOVA) and post hoc Tukey test. Relationships between the analyzed parameters were evaluated by computing Pearson linear correlation coefficients setting the level of significance at p < 0.05, p < 0.01, and p < 0.001 [11]. The statistical analysis was performed by SPSS 24.0. Multivariate analyses viz. principal component analysis (PCA) and hierarchical cluster analysis were performed with XLSTAT 2020 for Microsoft Excel 2016.
3. Results
3.1. Insect Flours Are a Sustainable Source of Protein, Chitin, and Lipids
Insect flours showed high content of protein (39.4%–58.1%) and fat (17.7%–50.1%) as main components, although the presence of chitin in ant samples was also highlighted (Figure 1A). The energy value depends on their two main components: protein and fat. Here, we observed a significant correlation (r = 0.999, p < 0.001; Figure 1B) between fat content and the insects’ energy value. Besides, insect flours exhibited high antioxidant capacity (179–221 mg Trolox equivalents/g), which was not associated with any chemical component measured.
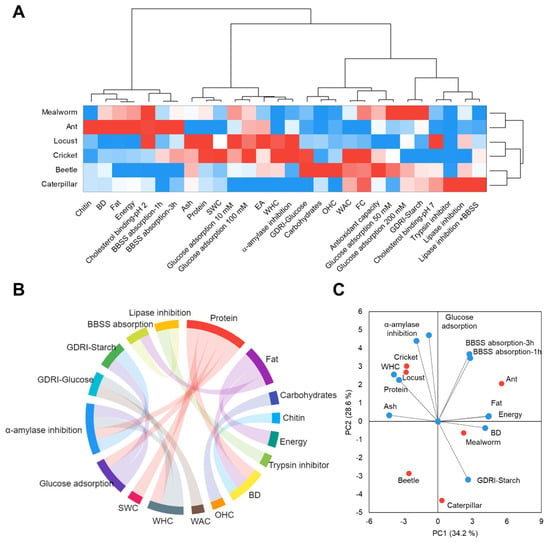
Figure 1.
Agglomerative hierarchical cluster analysis coupled to heat map (from the lowest (  ) to the highest (
) to the highest (  ) value for each parameter) showing the associations among the measured parameters and classifying insect flours according to them (A), chord diagram depicting the significant (p < 0.05) Pearson correlations (≥ |0.65|) between the chemical constituents of insect flours, techno-functional properties, and in vitro physiological properties (B), and Principal Component Analysis (PCA) representing each insect flour and the influence of the measured parameters on their classification (C). BD: Bulk density; WHC: water holding capacity; OHC: oil holding capacity; WAC: water absorption capacity; SWC: swelling capacity; EA: emulsifying activity; FC: foaming capacity; LGC: last gelation concentration; GDRI: glucose dialysis retardation index.
) value for each parameter) showing the associations among the measured parameters and classifying insect flours according to them (A), chord diagram depicting the significant (p < 0.05) Pearson correlations (≥ |0.65|) between the chemical constituents of insect flours, techno-functional properties, and in vitro physiological properties (B), and Principal Component Analysis (PCA) representing each insect flour and the influence of the measured parameters on their classification (C). BD: Bulk density; WHC: water holding capacity; OHC: oil holding capacity; WAC: water absorption capacity; SWC: swelling capacity; EA: emulsifying activity; FC: foaming capacity; LGC: last gelation concentration; GDRI: glucose dialysis retardation index.
 ) to the highest (
) to the highest (  ) value for each parameter) showing the associations among the measured parameters and classifying insect flours according to them (A), chord diagram depicting the significant (p < 0.05) Pearson correlations (≥ |0.65|) between the chemical constituents of insect flours, techno-functional properties, and in vitro physiological properties (B), and Principal Component Analysis (PCA) representing each insect flour and the influence of the measured parameters on their classification (C). BD: Bulk density; WHC: water holding capacity; OHC: oil holding capacity; WAC: water absorption capacity; SWC: swelling capacity; EA: emulsifying activity; FC: foaming capacity; LGC: last gelation concentration; GDRI: glucose dialysis retardation index.
) value for each parameter) showing the associations among the measured parameters and classifying insect flours according to them (A), chord diagram depicting the significant (p < 0.05) Pearson correlations (≥ |0.65|) between the chemical constituents of insect flours, techno-functional properties, and in vitro physiological properties (B), and Principal Component Analysis (PCA) representing each insect flour and the influence of the measured parameters on their classification (C). BD: Bulk density; WHC: water holding capacity; OHC: oil holding capacity; WAC: water absorption capacity; SWC: swelling capacity; EA: emulsifying activity; FC: foaming capacity; LGC: last gelation concentration; GDRI: glucose dialysis retardation index.
3.2. Insects Flours’ Physicochemical and Techno-Functional Properties Validated Their Use in Food Production
Insect flours displayed high oil holding, swelling, and emulsifying capacities in all insect flours analyzed, besides bulk density, hydration properties, and foaming capacity showing average values and no gelation capacity (Figure 1A). Bulk density was significantly correlated with the content of protein (r = −0.855, p < 0.05), fat (r = 0.858, p < 0.01), and chitin (r = 0.688, p < 0.05) (Figure 1B). On the other hand, oil holding capacity significantly correlated with carbohydrates’ content (r = 0.850, p < 0.05). Water holding and swelling capacities were significantly associated with the insect flours’ protein content (r = 0.784, p < 0.05 and r = 0.859, p < 0.01, respectively).
3.3. Insect Flours Could Retard Intestinal Glucose Absorption Reducing Postprandial Glycemia
Edible insect flours revealed effective hyperglycemic properties. Mealworm flour revealed the highest glucose binding at high glucose concentrations (Figure 1A). Glucose adsorption was associated with the protein content (r = 0.697, p < 0.01) (Figure 1B). Insect flours inhibited α-amylase activity (47.1%–8.0%). This inhibition exhibited a relationship with the protein content (r = 0.723, p < 0.05) and the water holding capacity (r = 0.800, p < 0.05). Likewise, insect flours retarded glucose diffusion (17.2%–29.6%) and starch hydrolysis (18.2%–88.1%). Glucose diffusion retardation correlated with the water absorption and holding capacities (r = 0.717, p < 0.05, and r = 0.703, p < 0.05, respectively). Consistently, the retardation of starch digestion was associated with the delaying of glucose diffusion (r = 0.832, p < 0.05).
3.4. Insect Flours Exhibited the Ability to Hinder Lipids Digestion Diminishing Their Absorption
Similarly, insect flours presented hypolipidemic properties. They bound cholesterol and bile salts (8.4%–98.6%) (Figure 1A). A moderate correlation of chitin content with bile salt absorption capacity was showed (r = 0.508, p < 0.05). A higher correlation was shown with the content of fat (r = 0.689, p < 0.05). Correspondingly, insect flour inhibited pancreatic lipase activity (8.9%–47.1%), which exhibited a significant association with the bile salt-binding capacity (r = 0.731, p < 0.05). A significant positive correlation was shown between the concentration of trypsin inhibitor and lipase inhibition (r = 0.811, p < 0.05).
3.5. Insect Flours Were Statistically Classified According to Their Composition and Properties
The statistical analysis enabled us to classify the insect flours according to their nutritional value, techno-functional, and in vitro physiological properties (Figure 1A,C). The agglomerative hierarchical cluster analysis and the PCA showed a first group, which clustered mealworm and ant flours, standing out by their fat and chitin content and their ability to bind to cholesterol and bile salts. A second group comprised locust and cricket flours, with higher protein content and, therefore, higher water holding, swelling, and emulsifying properties and higher capacity to adsorb glucose and inhibit α-amylase. The third group assembled beetle and caterpillar flours, with medium protein and chitin contents but a better ability to retard in vitro starch digestion and inhibit lipase activity. Thus, insects may be a great option to be used as a novel healthy ingredient.
4. Discussion
Cultural and religious practices heavily influence entomophagy. Insects are commonly consumed as a food source in many regions of the world. However, in most Western countries, people view entomophagy with disgust and associate eating insects with primitive behavior [12]. Despite this, the European Food Safety Authority (EFSA) has approved five different insect species as novel foods [13] since the demand for more sustainable food production has generated attention in insect consumption. To date, a few scattered studies have analyzed edible insects’ nutritional value; however, these data are not always comparable due to the variation between insects and the varying methodologies employed to analyze the chemical composition [14]. Here we present the assessment of the nutritional composition of six of the most consumed insects worldwide. As expected, a high variability on the protein, chitin, and lipid contents was observed. In this sense, edible insects’ nutritional value is highly variable because of the wide range of edible insect species. Even within the same group of species, nutritional value may differ depending on the insect’s metamorphic stage, the habitat in which it lives, and its diet [15]. Regarding insect flours’ physicochemical and techno-functional properties have been studied mostly in protein isolates, but the whole flour has been undervalued [16]. As sustainable foods, the easier and more cost-effective their obtention, the better. We propose their use as a complete ingredient, and thus, we assessed their functional properties to be included in food products. The variability in the insect flour chemical composition leads to a broad diversity of properties. As observed, techno-functional properties were associated with not only the protein content but with chitin, carbohydrates, and lipids. Similarly, insect flours’ health-promoting properties have been underestimated so far. A limited number of publications have focused on the association of insects’ chemical constituents and their potential biological activity [17]. This study reveals the hypoglycemic and hypolipidemic properties of six insect flours. Previously, research proved the ability of mealworm to reduce in vitro starch digestibility [18] and inhibit pancreatic lipase [19], and of cricket to lower LDL plasmatic cholesterol levels in rats [20]. Indeed, more research is needed to fully understand the potential of insects as functional foods or nutraceuticals. Future studies will unravel the biological activity of insects, and the mechanisms implied. Likewise, insects’ safety will be validated to ensure their use as a sustainable source of protein, chitin, and lipids, among other micronutrients and bioactive compounds.
5. Conclusions
This work presents a comprehensive analysis of the chemical composition, physicochemical, techno-functional, and in vitro physiological properties of six edible insect flours. Our findings confirm that insects are a sustainable source of protein, chitin, and lipids. Additionally, these flour foods may be valuable due to their physiological effects related to their hypoglycemic and hypolipidemic properties. These flours presented several applications due to their techno-functional properties. Upcoming works will extend the data presented in this study with the in vivo biological properties of insect flours, the technological, and the cooking applications.
Author Contributions
Conceptualization, Y.A.; methodology, Y.A., M.R.-H., and V.B.; formal analysis, M.R.-H.; investigation, I.P., M.R.-H., and G.A.-R.; resources, Y.A. and J.L.V.; data curation, M.R.-H.; writing—original draft preparation, Y.A. and M.R.-H.; writing—review and editing, Y.A., M.R.-H., V.B., and M.A.M.-C.; supervision, Y.A., M.R.-H., and M.A.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
M. Rebollo-Hernanz received funding from the Ministry of Universities FPU program for a pre-doctoral fellowship (FPU15/04238). G. Alvarez-Rivera received funding from the Ministry of Science and Innovation for a Juan de la Cierva postdoctoral grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Verbeke, W. Profiling consumers who are ready to adopt insects as a meat substitute in a Western society. Food Qual. Prefer. 2015, 39, 147–155. [Google Scholar] [CrossRef]
- Schlüter, O.; Rumpold, B.; Holzhauser, T.; Roth, A.; Vogel, R.F.; Quasigroch, W.; Vogel, S.; Heinz, V.; Jäger, H.; Bandick, N.; et al. Safety aspects of the production of foods and food ingredients from insects. Mol. Nutr. Food Res. 2017, 61, 1600520. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Ohara, A.; Aguilar, J.G.D.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis of. Anal. Chem. 1995, 52, 148A.
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the island of Sumatra. Int. J. Env. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Herrera, T.; Aguilera, Y.; Rebollo-Hernanz, M.; Bravo, E.; Benítez, V.; Martínez-Sáez, N.; Arribas, S.M.; del Castillo, M.D.; Martín-Cabrejas, M.A. Teas and herbal infusions as sources of melatonin and other bioactive non-nutrient components. Lwt Food Sci. Technol. 2018, 89, 65–73. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard reaction by phytochemicals composing an aqueous coffee silverskin extract via a mixed mechanism of action. Foods 2019, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- FAO. Edible Insects. Future Prospects for Food and feed Security; FAO: Roma, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- European Commission Summary of Ongoing Applications and Notifications Novel Food. Available online: https://ec.europa.eu/food/safety/novel_food/authorisations/summary-applications-and-notifications_en (accessed on 2 April 2021).
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Dossey, A.T. Insects and their chemical weaponry: New potential for drug discovery. Nat. Prod. Rep. 2010, 27, 1737–1757. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef] [PubMed]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).