Base Wine and Traditional Sparkling Wine Making Using Torulaspora delbrueckii Killer Yeasts †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Enhance of Killer T. delbrueckii Yeasts on the First Fermentation and Quality of Base Wine
3.2. Utility of T. delbrueckii on the Second Fermentation and the Quality of the Sparkling Wine
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, M.; Velázquez, R.; Maqueda, M.; Zamora, E.; López-Piñeiro, A.; Hernández, L.M. Influence of the dominance of must fermentation by Torulaspora delbrueckii on the malolactic fermentation and organoleptic quality of red table wine. Int. J. Food Microbiol. 2016, 238, 311–319. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2014, 240, 999–1012. [Google Scholar] [CrossRef]
- Brissonnet, F.; Maujean, A. Characterization of foaming proteins in a champagne base wine. Am. J. Enol. Vitic. 1993, 44, 297–301. [Google Scholar]
- Vanrell, G.; Canals, R.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava). Food Chem. 2007, 104, 148–155. [Google Scholar] [CrossRef]
- Gallart, M.; López-Tamames, E.; Suberbiola, G.; Buxaderas, S. Influence of fatty acids on wine foaming. J. Agric. Food Chem. 2002, 50, 7042–7045. [Google Scholar] [CrossRef] [PubMed]
- Todd, B.E.; Fleet, G.H.; Henschke, P.A. Promotion of autolysis through the interaction of killer and sensitive yeasts: Potential application in sparkling wine production. Am. J. Enol. Vitic. 2000, 51, 65–72. [Google Scholar]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Álvarez, M.L.; Ramírez, M. Using mixed inocula of new killer strains of Saccharomyces cerevisiae to improve the quality of traditional sparkling-wine. Food Microbiol. 2016, 59, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, R.; Zamora, E.; Alvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Using Torulaspora delbrueckii killer yeasts in the elaboration of base wine and traditional sparkling. Int. J. Food Microbiol. 2019, 289, 134–144. [Google Scholar] [CrossRef] [PubMed]
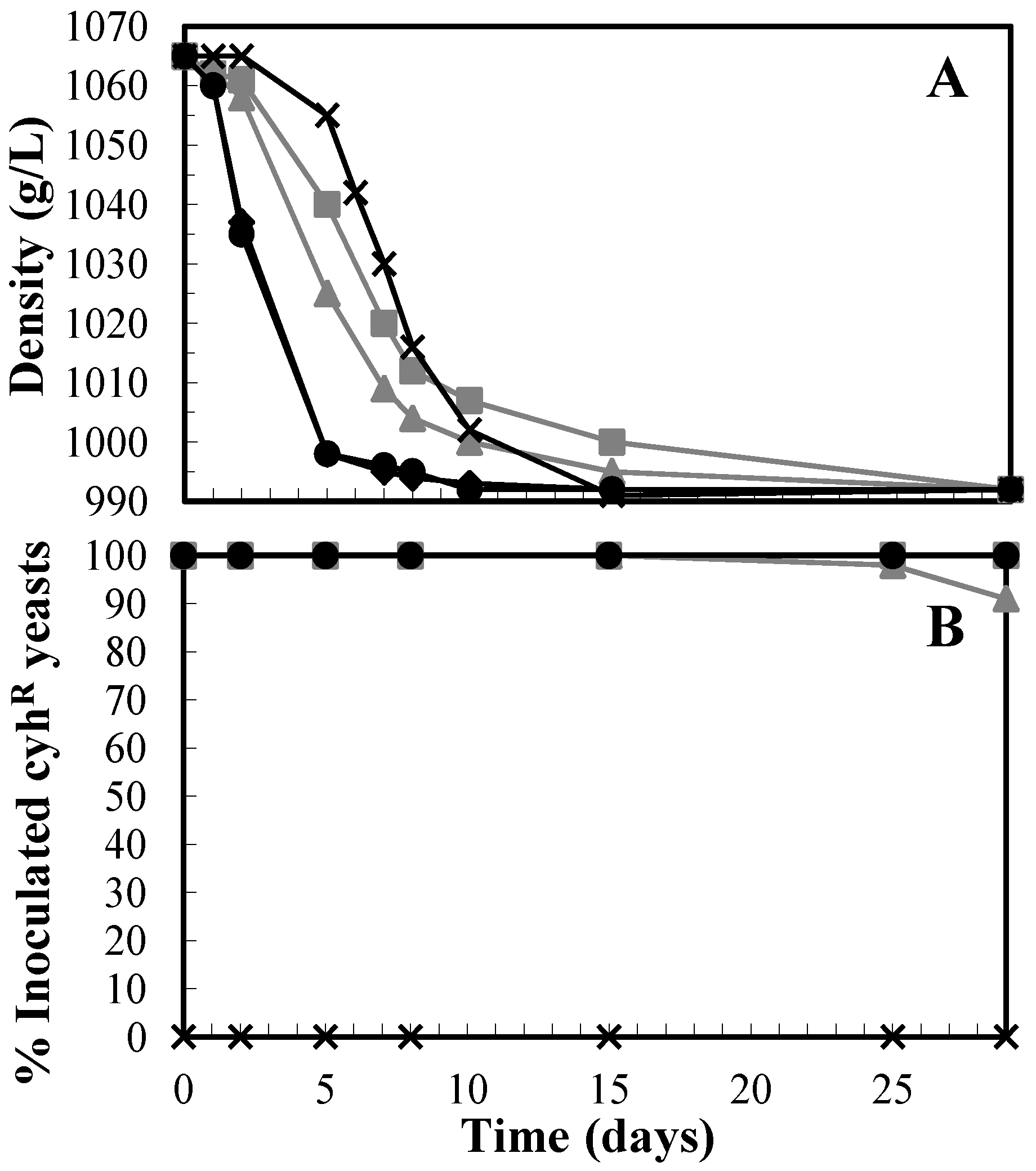
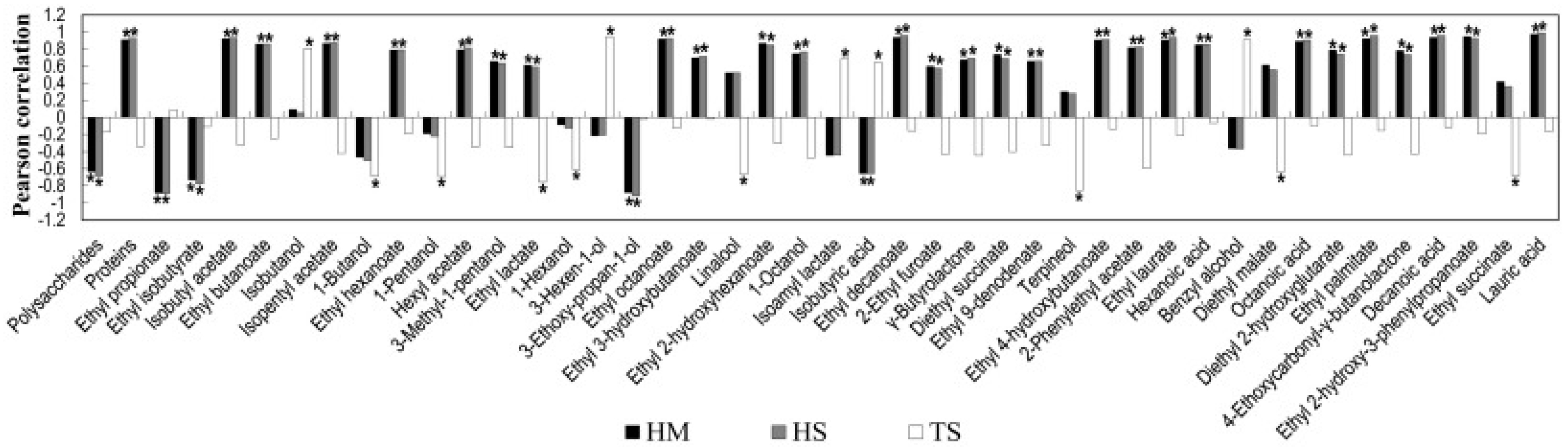
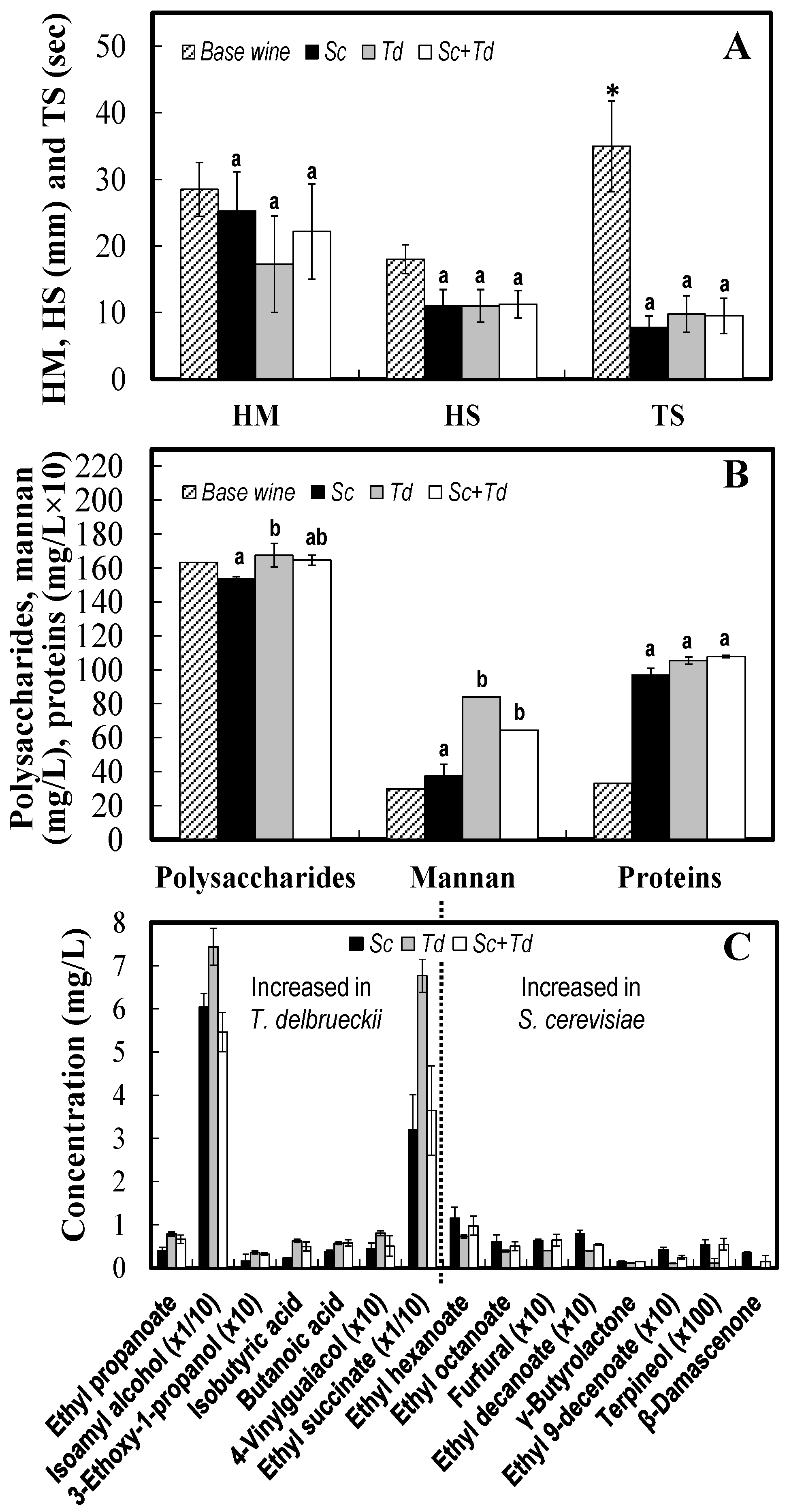
| Parameter | S. cerevisiae | T. delbrueckii | pa |
|---|---|---|---|
| T15 (days) | 1.58 ± 0.05 | 3.81 ± 0.3 | 0.000 |
| T100 (days) | 5.80 ± 0.5 | 18.2 ± 2.2 | 0.001 |
| Proportion at EF (%) | 100 ± 0.0 | 76.4 ± 17 | 0.205 |
| Alcohol (% v/v) | 10.5 ± 0.3 | 9.78 ± 0.4 | 0.206 |
| Reducing sugars (g/L) | 1.14 ± 0.1 | 6.46 ± 3.9 | 0.211 |
| Glycerol (g/L) | 6.1 ± 0.2 | 5.65 ± 0.3 | 0.315 |
| Polysaccharides (mg/L) | 150 ± 5 | 241 ± 32 | 0.000 |
| Proteins (mg/L) | 9.3 ± 0.4 | 6.2 ± 0.2 | 0.000 |
| Σ Ethyl esters (mg/L) | 19 ± 2.3 | 11 ± 1.8 | 0.027 |
| Σ Acetate esters (mg/L) | 167 ± 16 | 152 ± 18 | 0.542 |
| Σ Acids (mg/L) | 23 ± 1.2 | 7.3 ± 1.2 | 0.000 |
| Σ Alcohols (mg/L) | 153 ± 12 | 162 ± 16 | 0.652 |
| Σ Furans + phenols (mg/L) | 0.20 ± 0.07 | 0.09 ± 0.03 | 0.183 |
| HM (mm) | 174 ± 15 | 33 ± 3.7 | 0.000 |
| HS (mm) | 137 ± 8.7 | 19 ± 3.3 | 0.000 |
| TS (sec) | 111 ± 22 | 161 ± 33 | 0.248 |
| Parameter | S. cerevisiae | T. delbrueckii | Sc+Td | pa |
|---|---|---|---|---|
| Alcohol (%, v/v) | 11.4 ± 0.01a | 10.6 ± 0.15b | 11.3 ± 0.32a | 0.050 |
| pH | 3.16 ± 0.01a | 3.57 ± 0.04c | 3.28 ± 0.07b | 0.010 |
| Total acidity (g/L) | 5.82 ± 0.05a | 5.15 ± 0.05b | 5.35 ± 0.05b | 0.010 |
| Volatile acidity (g/L) | 0.27 ± 0.02a | 0.47 ± 0.01b | 0.44 ± 0.01b | 0.010 |
| Glucose + fructose (g/L) | 0.06 ± 0.0a | 7.4 ± 0.1b | 0.07 ± 0.01a | 0.000 |
| Density (g/L) | 989 ± 0.0a | 998 ± 0.0b | 992 ± 0.0a | 0.007 |
| Pressure (atm) | 6.1 ± 0.05a | 3.2 ± 0.90b | 6.05 ± 0.05a | 0.000 |
| Preference (%) | 65 ± 0.00a | 47 ± 1.50b | 78 ± 2.50c | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Velázquez, R.; Zamora, E.; Franco, M.L.; Garzo, C.; Gil, P.; Hernández, L.M.; Ramírez, M. Base Wine and Traditional Sparkling Wine Making Using Torulaspora delbrueckii Killer Yeasts. Proceedings 2021, 70, 69. https://doi.org/10.3390/foods_2020-07756
Martínez A, Velázquez R, Zamora E, Franco ML, Garzo C, Gil P, Hernández LM, Ramírez M. Base Wine and Traditional Sparkling Wine Making Using Torulaspora delbrueckii Killer Yeasts. Proceedings. 2021; 70(1):69. https://doi.org/10.3390/foods_2020-07756
Chicago/Turabian StyleMartínez, Alberto, Rocío Velázquez, Emiliano Zamora, María L. Franco, Camille Garzo, Patricia Gil, Luis M. Hernández, and Manuel Ramírez. 2021. "Base Wine and Traditional Sparkling Wine Making Using Torulaspora delbrueckii Killer Yeasts" Proceedings 70, no. 1: 69. https://doi.org/10.3390/foods_2020-07756
APA StyleMartínez, A., Velázquez, R., Zamora, E., Franco, M. L., Garzo, C., Gil, P., Hernández, L. M., & Ramírez, M. (2021). Base Wine and Traditional Sparkling Wine Making Using Torulaspora delbrueckii Killer Yeasts. Proceedings, 70(1), 69. https://doi.org/10.3390/foods_2020-07756





