Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly(Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Fabrication of Nanofibrous Meshes
2.2.2. Crosslinking Process
2.2.3. Fiber Diameters
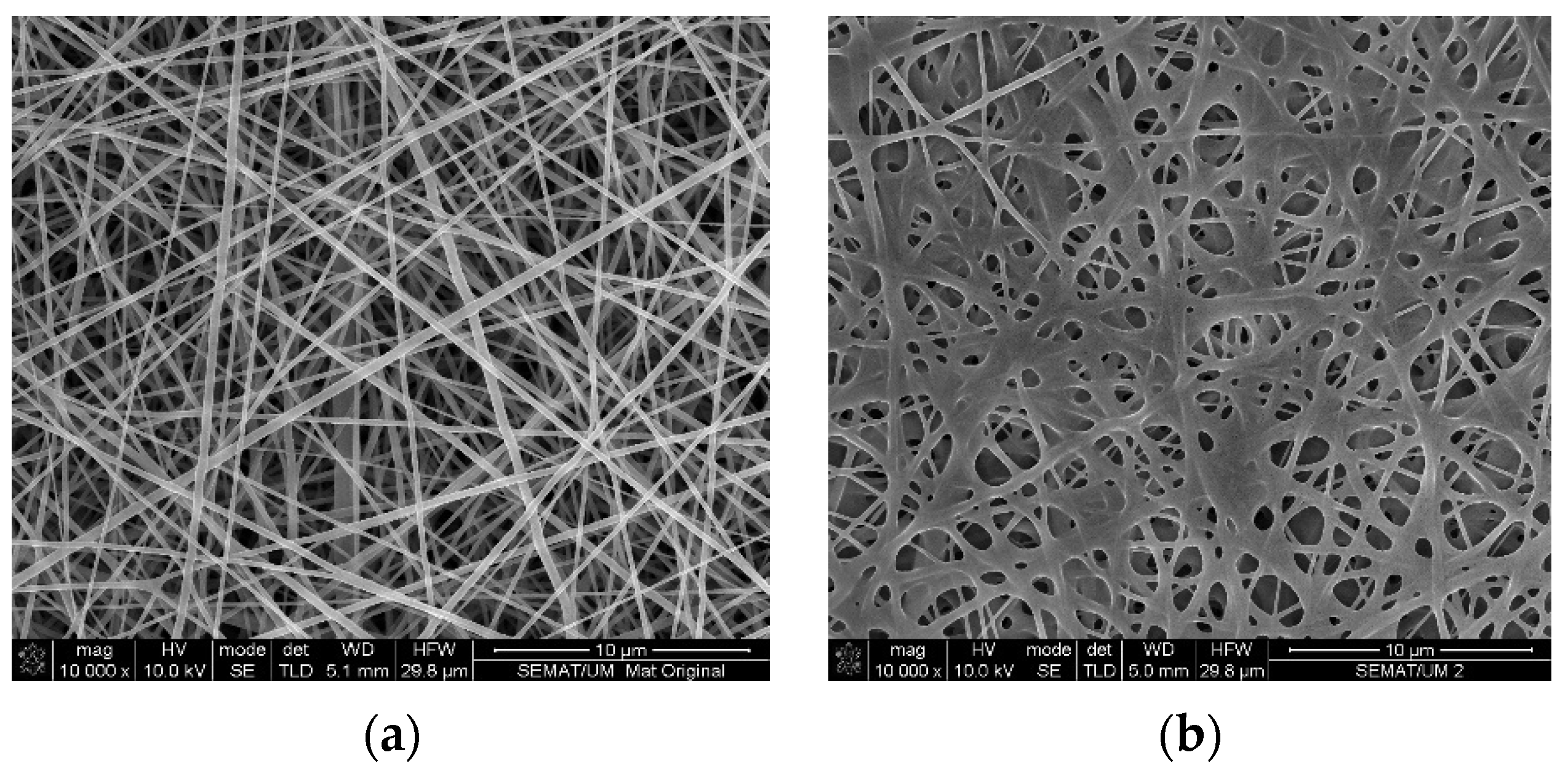
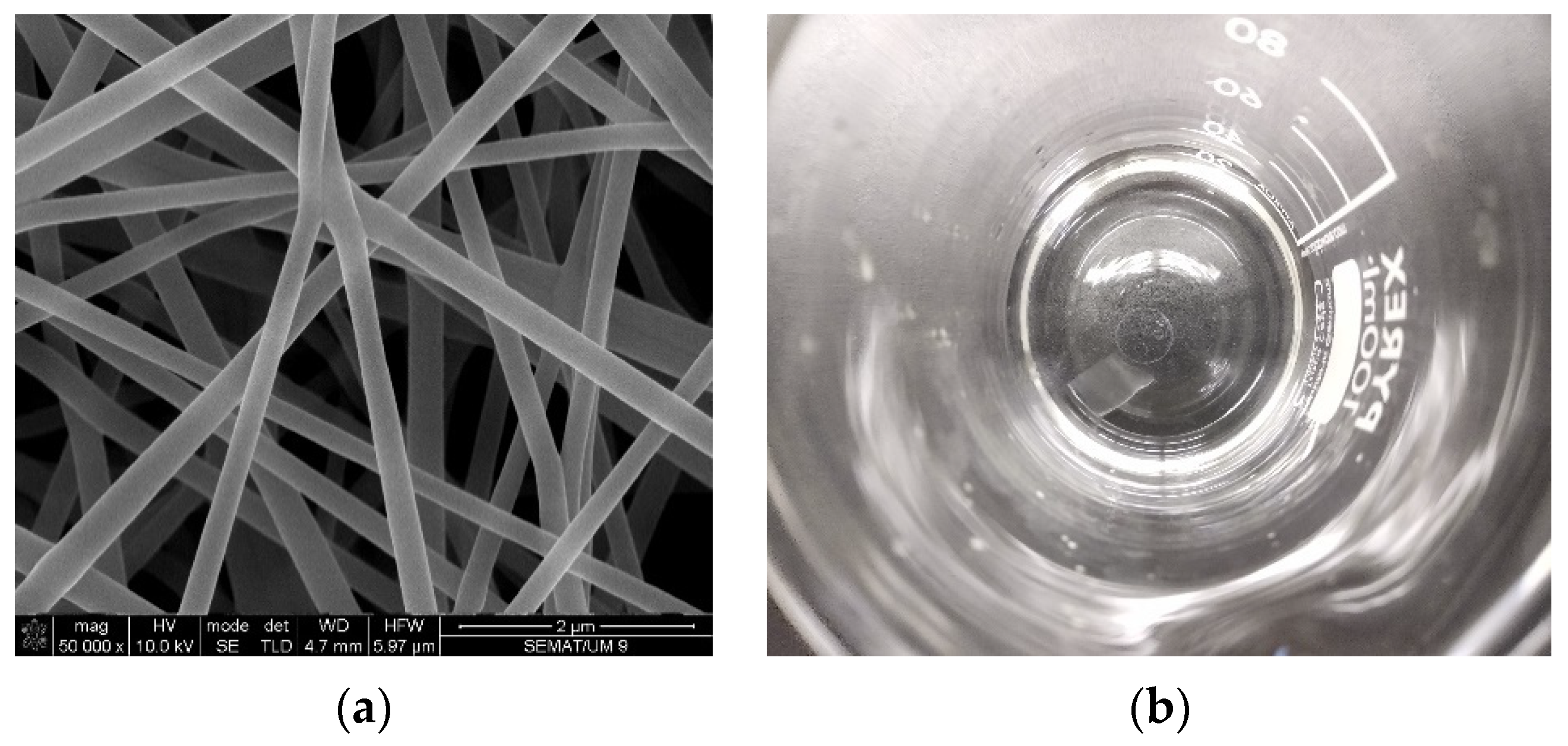
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Poly(vinyl alcohol) |
| CA | Cellulose acetate |
| CW | Chronic wounds |
| GA | Glutaraldehyde |
| RH | Relative humidity |
| RT | Room temperature |
References
- Kumar, A.; Behl, T.; Chadha, S. A rationalized and innovative perspective of nanotechnology and nanobiotechnology in chronic wound management. J. Drug Deliv. Sci. Technol. 2020, 60, 101930. [Google Scholar] [CrossRef]
- Las, K.; Igartua, M.; Santos-vizcaino, E.; Maria, R. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2018, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Mirzadegan, E.; Golshahi, H.; Kazemnejad, S. Current evidence on immunological and regenerative effects of menstrual blood stem cells seeded on scaffold consisting of amniotic membrane and silk fibroin in chronic wound. Int. Immunopharmacol. 2020, 85, 106595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Cellulose Acetate in Wound Dressings Formulations: Potentialities and Electrospinning Capability. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing–MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; Springer International Publishing: Cham, Switzerland, 2020; Volume 76, pp. 1515–1525. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C 2019, 105, 110083. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Li, Y.; Zhao, R.; Zhang, L.; Li, Y.; Wang, C.; Li, X. Synthesis and characterization of tigecycline-loaded sericin/poly(vinyl alcohol) composite fibers via electrospinning as antibacterial wound dressings. J. Drug Deliv. Sci. Technol. 2018, 44, 440–447. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly (Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.P. Physical, thermal, and antibacterial effects of active essential oils with potential for biomedical applications loaded onto cellulose acetate/polycaprolactone wet-spun microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly (vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. Appl. Polym. 2019, 137, 48626. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Destaye, A.G.; Lin, C.; Lee, C. Glutaraldehyde Vapor Cross-linked Nanofibrous PVA Mat with in Situ Formed Silver Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Pruthi, V. Synthesis and characterization of crosslinked gellan/PVA nanofibers for tissue engineering application. Mater. Sci. Eng. C 2016, 67, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. PVA/CA based electrospun nanofibers: Influence of processing parameters in the fiber diameter. IOP Conf. Ser. Mater. Sci. Eng. 2019, 634, 012040. [Google Scholar] [CrossRef]
| Process | Sonication | Orbital Shaker | Glycine |
|---|---|---|---|
| Duration (min) | 15 vs. 30 | 15 vs. 30 | 15 |
| Structure (treatment time or glycine concentration) |  (15 min) |  (15 min) |  (0.5% v/v) |
 (30 min) |  (30 min) |  (2.0% v/v) | |
| Temperature (°C) | RT | 37 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.A.; Antunes, J.C.; Amorim, M.T.P.; Felgueiras, H.P. Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly(Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings. Proceedings 2021, 69, 30. https://doi.org/10.3390/CGPM2020-07193
Teixeira MA, Antunes JC, Amorim MTP, Felgueiras HP. Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly(Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings. Proceedings. 2021; 69(1):30. https://doi.org/10.3390/CGPM2020-07193
Chicago/Turabian StyleTeixeira, Marta A., Joana C. Antunes, M. Teresa P. Amorim, and Helena P. Felgueiras. 2021. "Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly(Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings" Proceedings 69, no. 1: 30. https://doi.org/10.3390/CGPM2020-07193
APA StyleTeixeira, M. A., Antunes, J. C., Amorim, M. T. P., & Felgueiras, H. P. (2021). Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly(Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings. Proceedings, 69(1), 30. https://doi.org/10.3390/CGPM2020-07193







