Characterization of Lactobacillus brevis with Potential Probiotic Properties and Biofilm Inhibition against Pseudomonas aeruginosa †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of Lactic Acid Bacteria
2.2. Determination of Tolerance to Sodium Chloride, Bile Salt, Low pH, and Lysozyme
2.3. Antibiotic Susceptibility Test
2.4. Antimicrobial Activity
2.5. Cell Surface Hydrophobicity
2.6. Antibiofilm Activity
2.7. Stability at Two Different Temperatures
2.8. Stability in Ice Cream
3. Results
3.1. Screening, Isolation, and Identification of Lactic Acid Bacteria
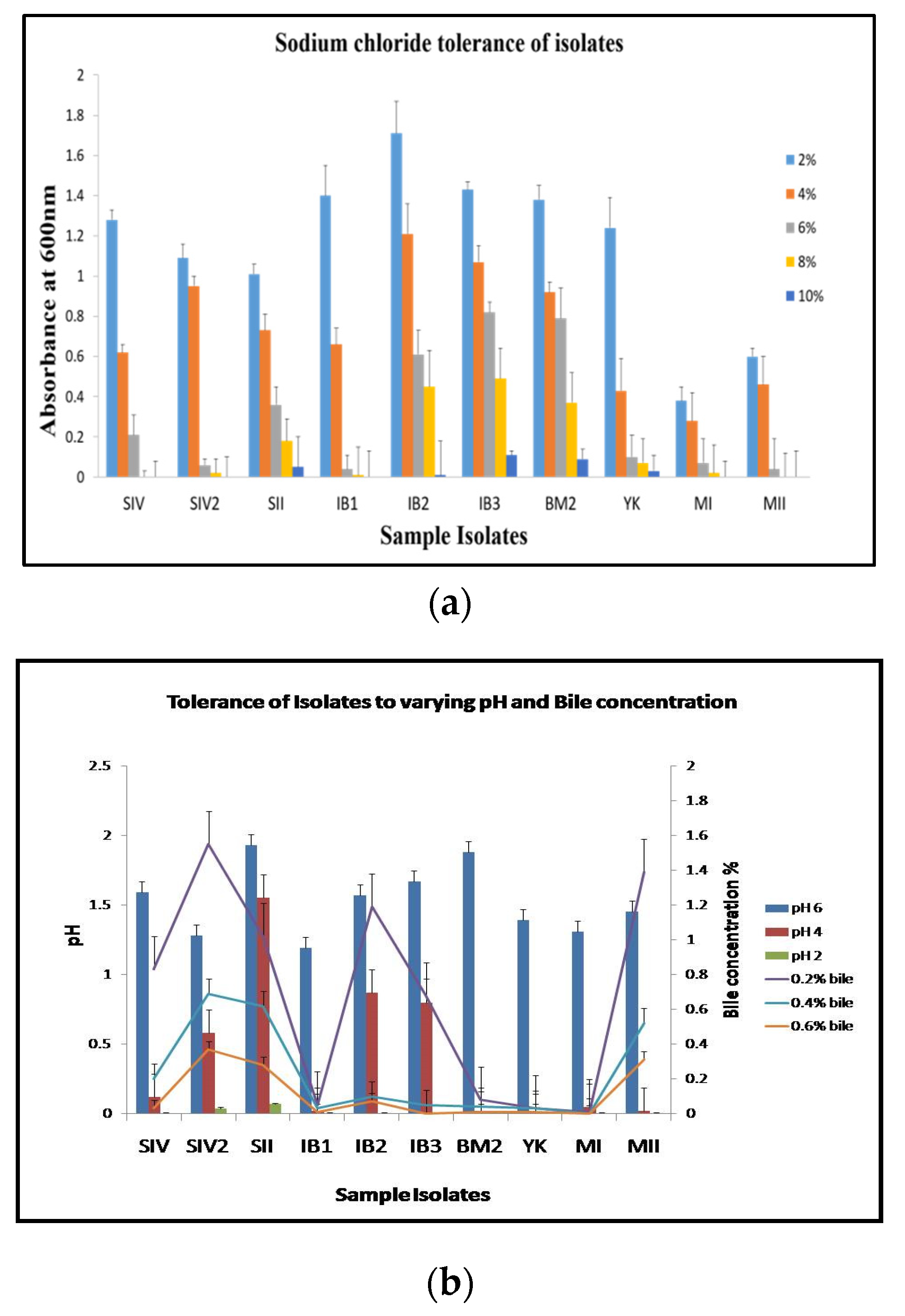
3.2. Tolerance to Sodium Chloride, Bile Salts, Low pH, and Lysozyme
3.3. Antibiotic Susceptibility Test
3.4. Antagonistic Activity Against Pathogens
3.5. Cell Surface Hydrophobicity
3.6. Antibiofilm Activity of Isolate SII
3.7. Stability of L.brevis at Two Different Temperatures
3.8. Stability of L.brevis in IceCream
3.9. Molecular and Genetic Analysis of SII
4. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gismondo, M.; Drago, L.; Lombardi, A. Review of Probiotics Available to Modify Gastrointestinal Flora. Antimicrob. Infect. Dis. Newsl. 1998, 17, 79. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Jairath, S. Prebiotics, probiotics, and synbiotics: An Overview. J. Pharm. Educ. Res. 2010, 1, 19–25. [Google Scholar]
- Foligné, B.; Daniel, C.; Pot, B. Probiotics from research to market: The possibilities, risks and challenges. Curr. Opin. Microbiol. 2013, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An Overview of Beneficial Effects. Lact. Acid Bact.Genet. Metab. Appl. 2002, 279–289. [Google Scholar] [CrossRef]
- Ohara, M.A.; Shanahan, F. Mechanisms of Action of Probiotics in Intestinal Diseases. Sci. World J. 2007, 7, 31–46. [Google Scholar] [CrossRef]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Caplice, E. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Quinto, E.J.; Jimenez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbes, T. Probiotic lactic acid bacteria: A review. Food Nutr. Sci. 2014, 5, 1765–1775. [Google Scholar] [CrossRef]
- Kandler, O.; Weiss, N. Regular, nonsporing gram-positive rods. In Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1986; Volume 2, pp. 1208–1260. [Google Scholar]
- O’Sullivan, M.G.; Thornton, G.; O’Sullivan, G.C.; Collins, J.K. Probiotic bacteria: Myth or reality? Trends Food Sci. Technol. 1992, 3, 309–314. [Google Scholar] [CrossRef]
- Collins, J.K.; Thornton, K.; Sullivan, G.O. Selection of probiotic strains for human applications. Int. Dairy J. 1998, 8, 487–490. [Google Scholar] [CrossRef]
- Kishi, A.; Kazuko, U.; Matsubara, Y.; Okuda, C.; Kishida, T. Effect of oral administration of Lactobacillus brevis subsp. Coagulans on interferon-a producing capacity of humans. J. Am. Coll. Nutr. 1996, 15, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The Inhibition Effect of Lactobacilli Against Growth and Biofilm Formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2017, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Anju, C.; Biswas, L.; Kumar, V.A.; Mohan, C.G.; Biswas, R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 2015, 306, 48–58. [Google Scholar] [CrossRef]
- Raras, T.Y.M.; Firdausy, A.F.; Kinanti, I.R.; Noorhamdani, N. Anti-Biofilm Activity of Lactic Acid Bacteria Isolated from Kefir Against Multidrug-Resistant Klebsiella pneumonia. J. Pure Appl. Microbiol. 2019, 13, 983–992. [Google Scholar] [CrossRef]
- Hartemink, R.; Domenech, V.R.; Rombouts, F.M. LAMVAB—A New Selective Medium for the Isolation of Lactobacilli from Faeces. J. Microbiol. Methods 1997, 29, 77–84. [Google Scholar] [CrossRef]
- Sneath, P.H.E.; Mair, N.S.E.; Sharpe, M.E.E.; Holt, J.G.E. Bergeys Manual ofSystematic Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1986; Volume 2. [Google Scholar]
- Ouwehand, A.; Vesterlund, S. Antimicrobial Components from Lactic Acid Bacteria. Lactic Acid Bact. 2004, 139, 375–396. [Google Scholar]
- De Valdez, G.F.; Taranto, M. Probiotic Properties of Lactobacilli: Cholesterol Reduction and Bile Salt Hydrolase Activity. Food Microbiol. Protoc. 2001, 173–181. [Google Scholar] [CrossRef]
- Prabhurajeshwar, C.; Chandrakanth, R.K. Probiotic Potential of Lactobacilli with Antagonistic Activity against Pathogenic Strains: An in Vitro Validation for the Production of Inhibitory Substances. Biomed. J. 2017, 40, 270–283. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Suzuki, C.; Kobayashi, M.; Mizumachi, K. Different Growth Media Alter the Induction of Interleukin 12 by a Lactococcus Lactis Strain. J. Food Prot. 2008, 71, 2124–2128. [Google Scholar] [CrossRef]
- Gillies, R.R.; Govan, J.R.W. Typing of Pseudomonas Pyocyanea by Pyocine Production. J. Pathol. Bacteriol. 1966, 91, 339–345. [Google Scholar] [CrossRef]
- Klayraung, S. Probiotic properties of Lactobacilli Isolated from Thai Traditional Food. Sci. Pharm. 2008, 76, 485–503. [Google Scholar] [CrossRef]
- Mathkhury, H.J.F.; Ali, A.S.; Ghafil, J.A. Antagonistic Effect of Bacteriocin against Urinary Catheter Associated Pseudomonas Aeruginosa Biofilm. N. Am. J. Med Sci. 2011, 367–370. [Google Scholar] [CrossRef]
- Chou, L.; Weimer, B. Isolation and Characterization of Acid- and Bile-Tolerant Isolates from Strains of Lactobacillus acidophilus. J. Dairy Sci. 1999, 82, 23–31. [Google Scholar] [CrossRef]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and Characterisation of Lactobacillus and Bifidobacterium Strains for Use as Probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. 1998, 61, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Rönkä, E.; Erja, M.; Maria, S.; Merja, R.K.; Johannes, A.; Airi, P. Probiotic and Milk Technological Properties of Lactobacillus Brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus Brevis Strain with Potential Oral Probiotic Properties. BMC Microbiol. 2018, 18. [Google Scholar] [CrossRef]
- Mathara, J.; Schillinger, U.; Guigas, C.; Franz, C.; Mbugua, S.K.; Shin, H.K.; Holzapfel, W.H. Functional Characteristics of Lactobacillus Spp. from Traditional Maasai Fermented Milk Products in Kenya. Int. J. Food Microbiol. 2008, 126, 57–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Ganger, S.; Patil, S. Characterization of Lactobacillus brevis with Potential Probiotic Properties and Biofilm Inhibition against Pseudomonas aeruginosa . Proceedings 2020, 66, 14. https://doi.org/10.3390/proceedings2020066014
Singh V, Ganger S, Patil S. Characterization of Lactobacillus brevis with Potential Probiotic Properties and Biofilm Inhibition against Pseudomonas aeruginosa . Proceedings. 2020; 66(1):14. https://doi.org/10.3390/proceedings2020066014
Chicago/Turabian StyleSingh, Vaishali, Suman Ganger, and Shweta Patil. 2020. "Characterization of Lactobacillus brevis with Potential Probiotic Properties and Biofilm Inhibition against Pseudomonas aeruginosa " Proceedings 66, no. 1: 14. https://doi.org/10.3390/proceedings2020066014
APA StyleSingh, V., Ganger, S., & Patil, S. (2020). Characterization of Lactobacillus brevis with Potential Probiotic Properties and Biofilm Inhibition against Pseudomonas aeruginosa . Proceedings, 66(1), 14. https://doi.org/10.3390/proceedings2020066014



