Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%) †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acid-Free Synthesis of Bioactive Glasses Containing ZnO
2.2. In Vitro Test in SBF Solution
2.3. In Vitro Test with Cell Culture
2.4. Physical-Chemical Characterization
3. Results and Discussion
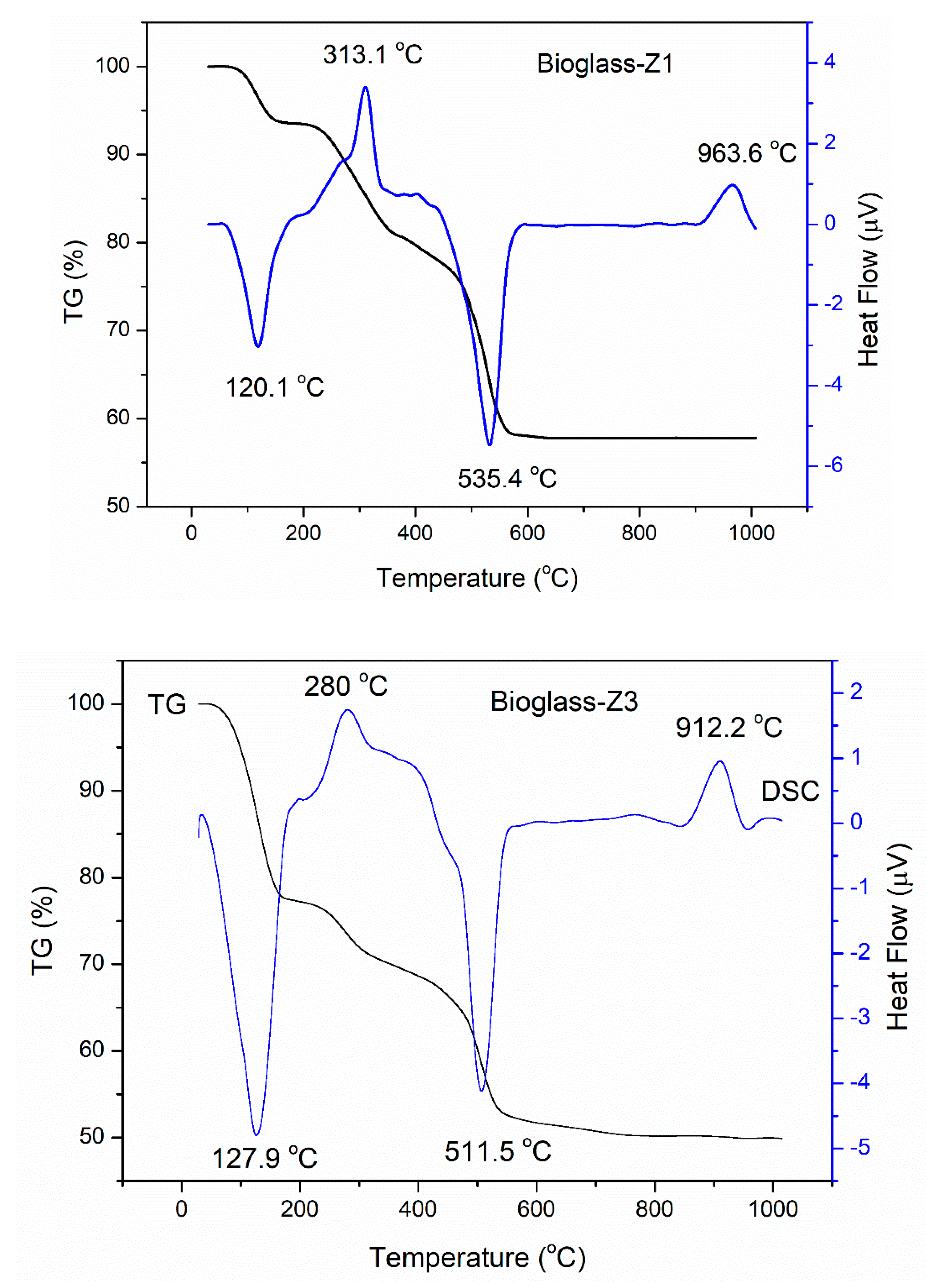
3.1. Thermal Behavior
3.2. Phase Analysis
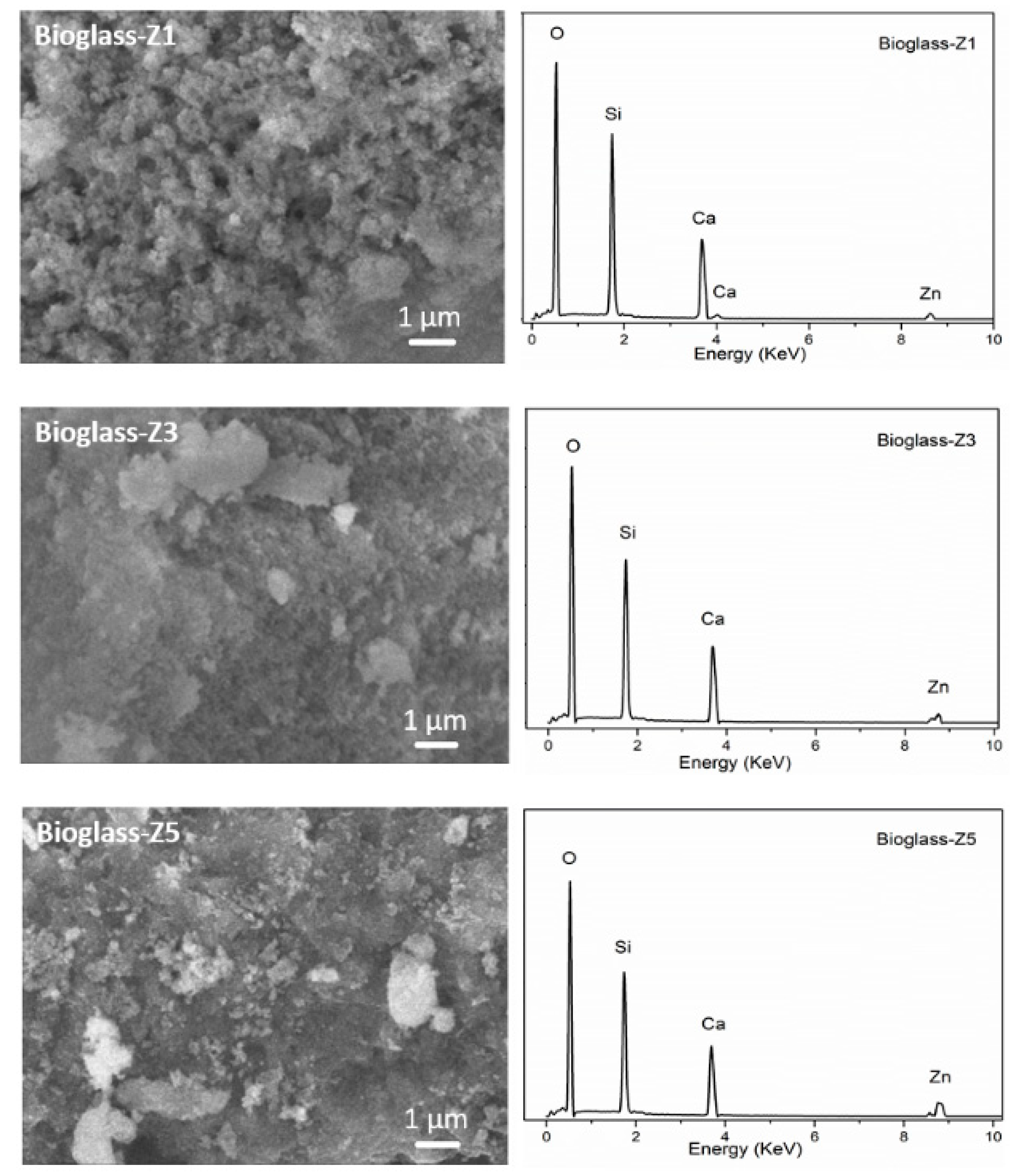
3.3. Textural Characterization
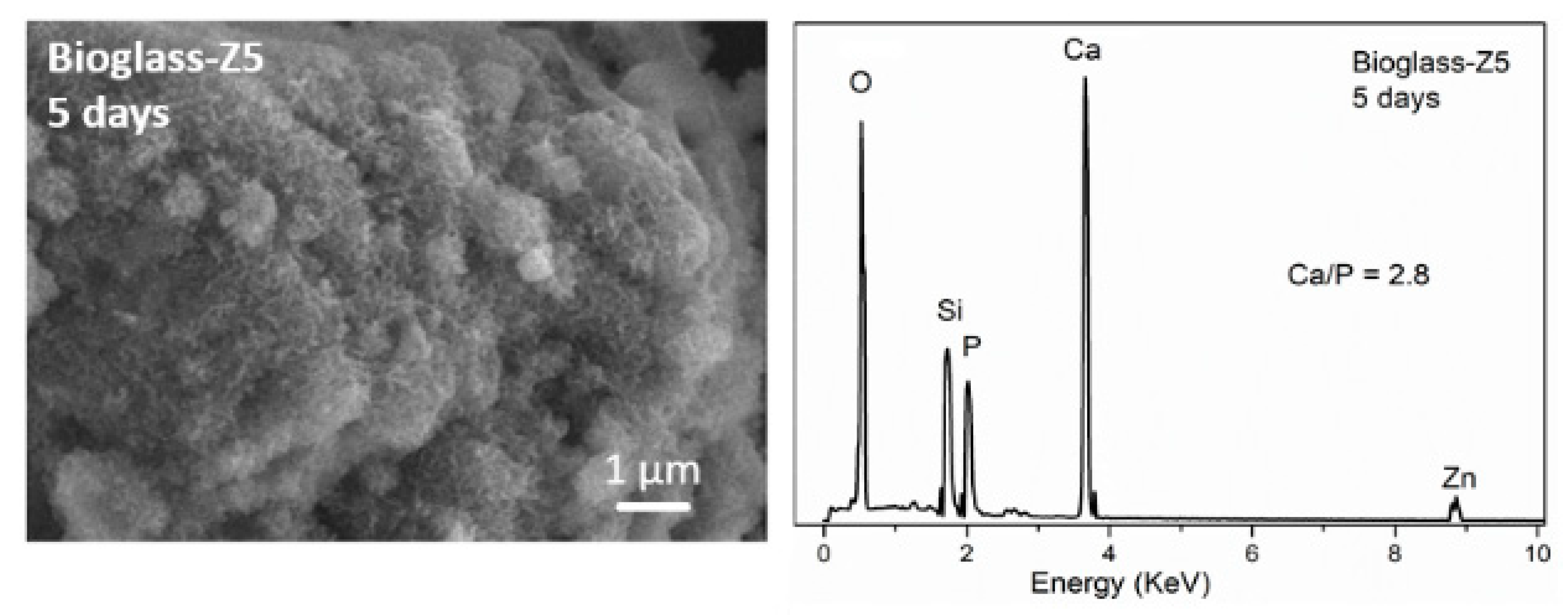
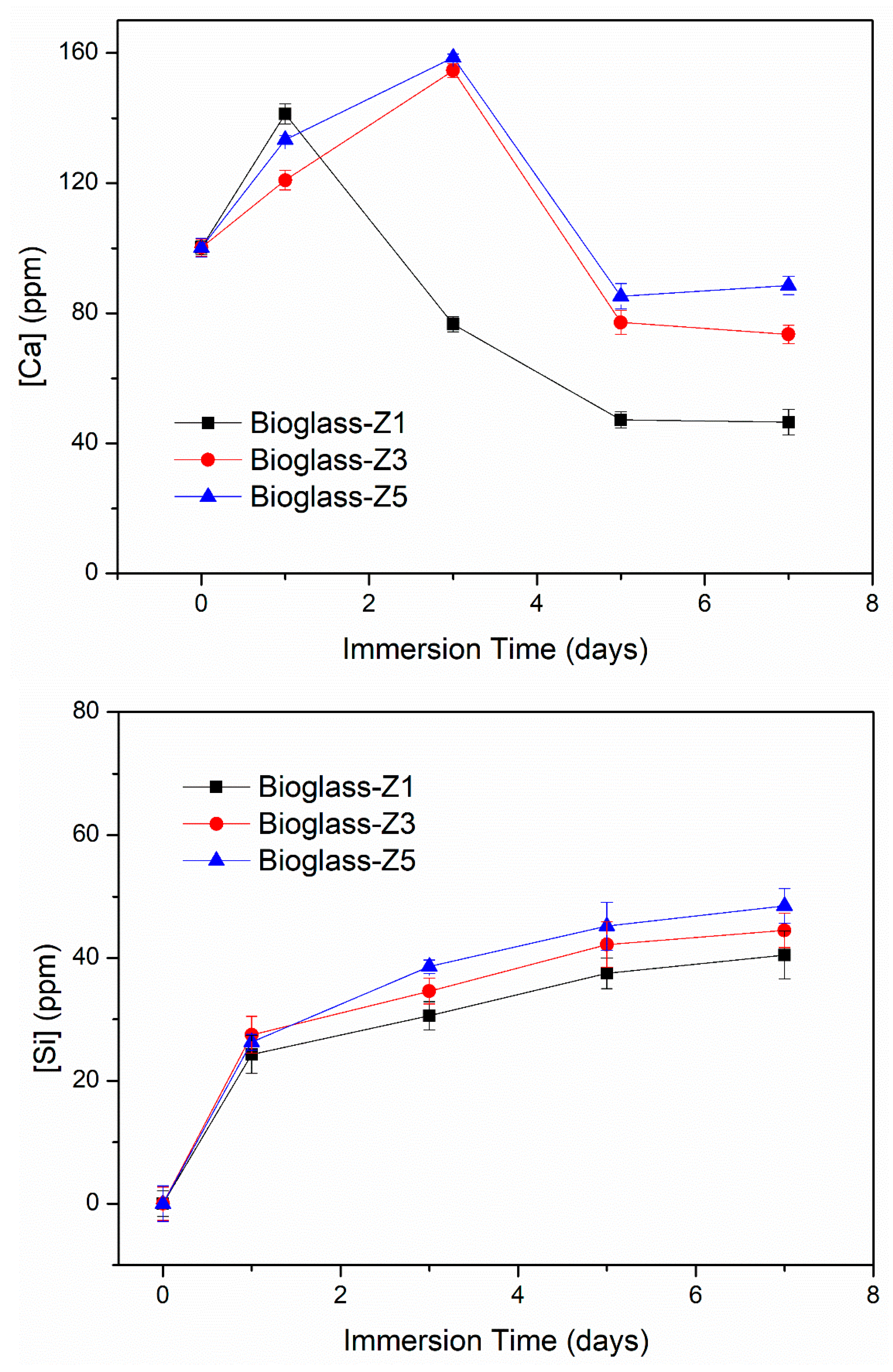
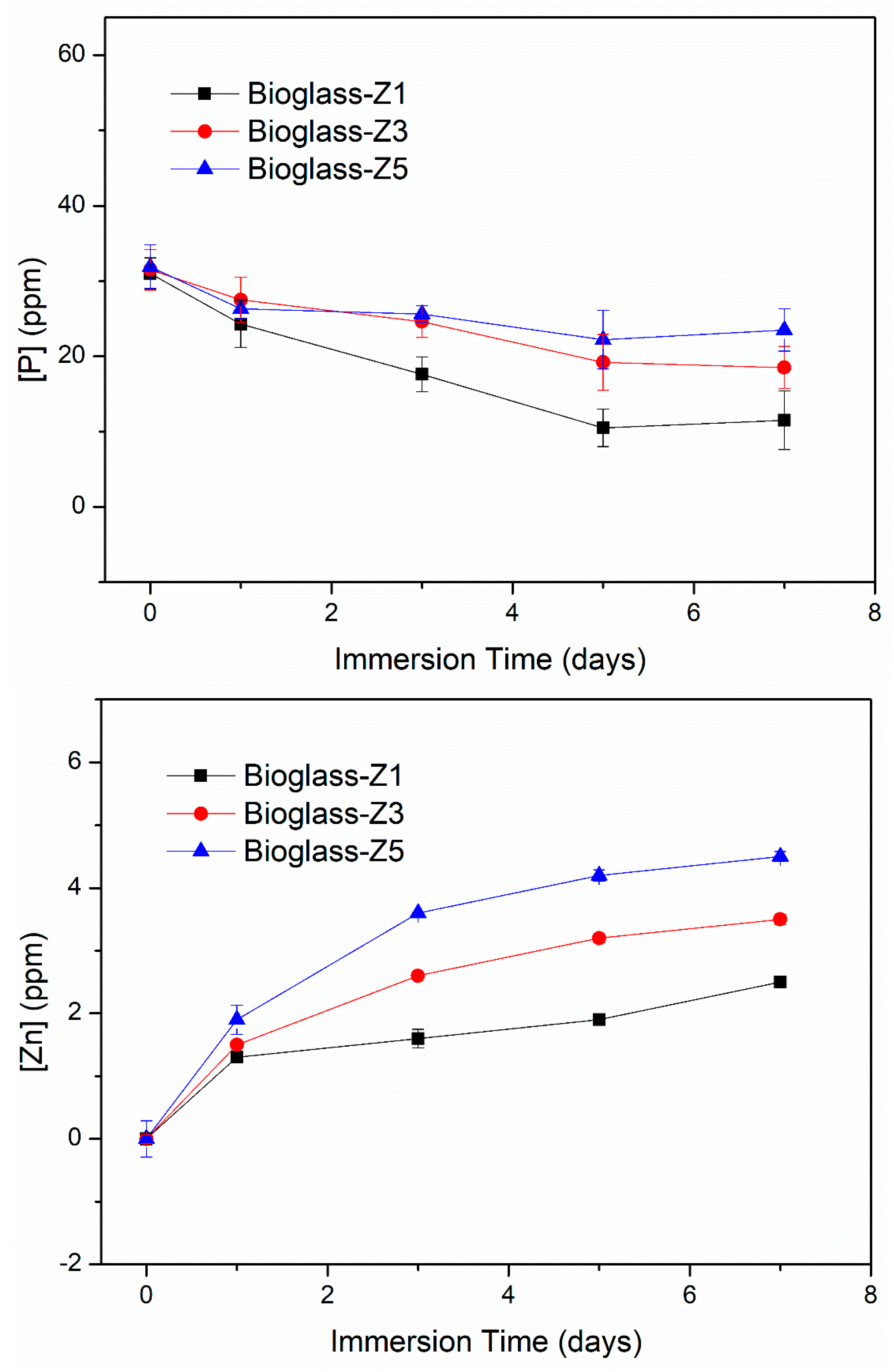
3.4. Bio-Mineralization
3.5. Biocompatibility
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Act. Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alquaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Coll. Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazia, F.; Parvina, N.; Tahriri, M. Synthesis and characteristics of sol-gel bioactive SiO2-P2O5-CaO-Ag2O glasses. J. Non-Cryst. Solids 2017, 476, 108–113. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balamurugan, A.; Balossier, G.; Kannan, S.; Michel, J.; Rebelo, A.H.; Ferreira, J.M. Development and in vitro characterization of sol-gel derived CaO-P2O5-SiO2-ZnO bioglass. Act. Biomater. 2007, 3, 255–262. [Google Scholar] [CrossRef]
- Salman, S.; Salama, S.; Mosallam, H.A. The role of strontium and potassium on crystallization and bioactivity of Na2O-CaO-P2O5-SiO2 glasses. Ceram. Int. 2012, 38, 55–63. [Google Scholar] [CrossRef]
- Kheshen, A.E.; Khaliafa, F.A.; Saad, E.A.; Elwan, R.L. Effect of Al2O3 addition on bioactivity, thermal and mechanical properties of some bioactive glasses. Ceram. Int. 2008, 34, 1667–1673. [Google Scholar] [CrossRef]
- Errol, M.; Özyuguran, A.; Çelebican, O. Synthesis, Characterization, and In Vitro Bioactivity of Sol-Gel-Derived Zn, Mg, and Zn-Mg Co-Doped Bioactive Glasses. Chem. Eng. Technol. 2010, 33, 1066–1074. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Act. Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.R.; Love, B.J.; Goldstein, A.S. Effect of soluble zinc on differentiation of osteoprogenitor cells. J. Biomed. Mater. Res. Part A 2007, 81, 66–769. [Google Scholar] [CrossRef] [PubMed]
- E-Kady, A.E.; Ali, A.F. Fabrication and characterization of ZnO modified bioactive glass nanoparticles. Ceram. Int. 2012, 38, 1195–1204. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef]
- Hoa, B.T.; Hoa, H.T.T.; Tien, N.A.; Khang, N.H.D.; Guseva, E.V.; Tuan, N.A.; Vuong, B.X. Green synthesis of bioactive glass 70SiO2-30CaO by hydrothermal method. Mater. Lett. 2020, 274, 128032. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J.J. Preparation and characterization of nano-bioactiveglasses (NBG) by a quick alkali-mediated (sol-gel) method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non-Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Román, J.; Padilla, S.; Vallet-Regí, M. Sol-gel glasses as precursors of bioactive glass ceramics. Chem. Mater. 2003, 15, 798–806. [Google Scholar] [CrossRef]
- Courthéoux, L.; Lao, J.; Nedelec, J.M.; Jallot, E. Controlled bioactivity in zinc-doped sol-gelderived binary bioactive glasses. J. Phys. Chem. C 2008, 112, 13663–13667. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol–gel derived CaO-P2O5-SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Oudadesse, H.; Dietrich, E.; Gal, Y.L.; Pellen, P.; Bureau, B.; Mostafa, A.A.; Cathelineau, G. Apatite forming ability and cytocompatibility of pure and Zn-doped bioactive glasses. Biomed. Mater. 2011, 6, 1–9. [Google Scholar] [CrossRef]





| Samples | Specific Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| Bioglass-Z1 | 133.6 | 20.8 | 0.78 |
| Bioglass-Z3 | 109.5 | 18.4 | 0.51 |
| Bioglass-Z5 | 74.9 | 18.2 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuan, T.A.; Guseva, E.V.; Phuc, L.H.; Hien, N.Q.; Long, N.V.; Vuong, B.X. Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%). Proceedings 2020, 62, 6. https://doi.org/10.3390/proceedings2020062006
Tuan TA, Guseva EV, Phuc LH, Hien NQ, Long NV, Vuong BX. Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%). Proceedings. 2020; 62(1):6. https://doi.org/10.3390/proceedings2020062006
Chicago/Turabian StyleTuan, Ta Anh, Elena V. Guseva, Le Hong Phuc, Nguyen Quan Hien, Nguyen Viet Long, and Bui Xuan Vuong. 2020. "Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%)" Proceedings 62, no. 1: 6. https://doi.org/10.3390/proceedings2020062006
APA StyleTuan, T. A., Guseva, E. V., Phuc, L. H., Hien, N. Q., Long, N. V., & Vuong, B. X. (2020). Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%). Proceedings, 62(1), 6. https://doi.org/10.3390/proceedings2020062006






