Polyproline-Rich Peptides Organize Four Cholinesterase Subunits into a Tetramer; BChE and AChE Scavenge Polyproline Peptides Released during Metabolic Turnover †
Abstract
:1. Introduction
2. Tetramers Are the Product of More Than One Gene
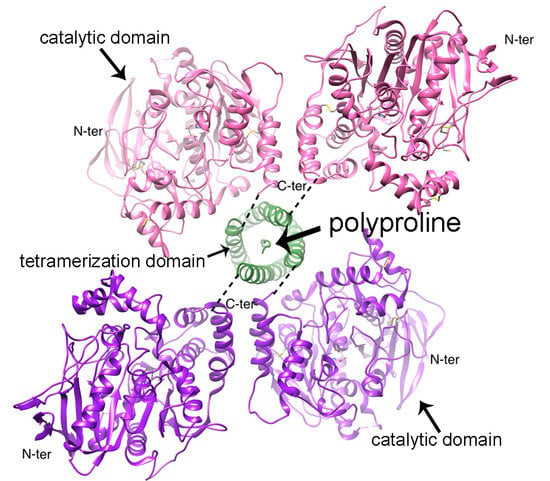
3. Tetramerization Domain
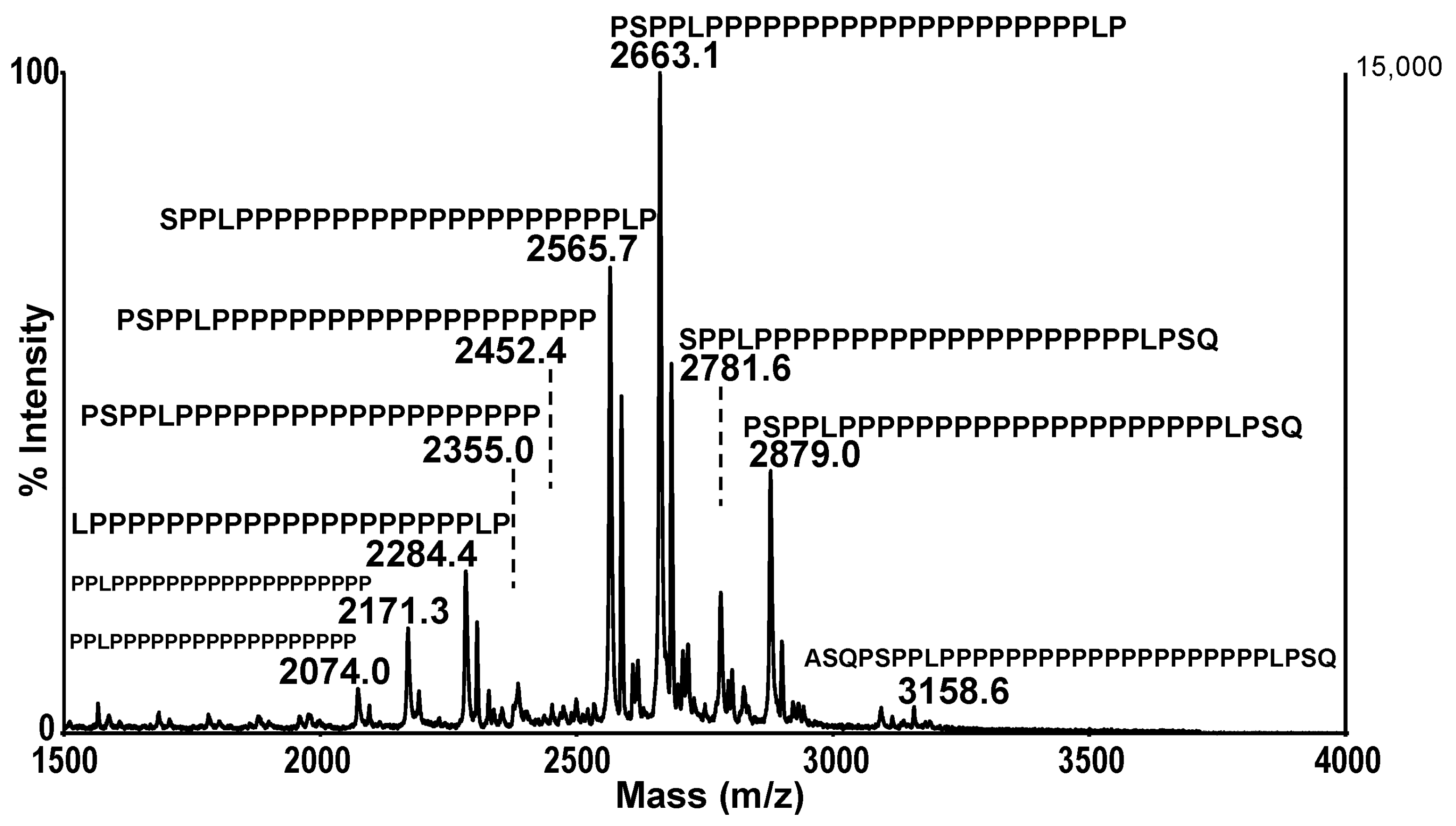
4. Mass Spectrometry Identification of Tetramer Organizing Peptides
5. Polyproline-Rich Peptides in Soluble BChE Tetramers
6. Polyproline-Rich Peptides in Soluble AChE Tetramers
7. BChE and AChE Scavenge Polyproline Peptides Released from Proteins in the Cytoplasm, Nucleus, Endoplasmic Reticulum, Extracellular Space, and Cell Membrane
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostergaard, D.; Viby-Mogensen, J.; Hanel, H.K.; Skovgaard, L.T. Half-life of plasma cholinesterase. Acta Anaesthesiol. Scand. 1988, 32, 266–269. [Google Scholar] [CrossRef]
- Kolarich, D.; Weber, A.; Pabst, M.; Stadlmann, J.; Teschner, W.; Ehrlich, H.; Schwarz, H.P.; Altmann, F. Glycoproteomic characterization of butyrylcholinesterase from human plasma. Proteomics 2008, 8, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O.; Bartels, C.F.; Vaughan, T.A.; Wong, C.K.; Norton, S.E.; Johnson, L.L. Complete amino acid sequence of human serum cholinesterase. J. Biol. Chem. 1987, 262, 549–557. [Google Scholar] [CrossRef]
- Leung, M.R.; van Bezouwen, L.S.; Schopfer, L.M.; Sussman, J.L.; Silman, I.; Lockridge, O.; Zeev-Ben-Mordehai, T. Cryo-EM structure of the native butyrylcholinesterase tetramer reveals a dimer of dimers stabilized by a superhelical assembly. Proc. Natl. Acad. Sci. USA 2018, 115, 13270–13275. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Krejci, E.; Massoulie, J. A four-to-one association between peptide motifs: Four C-terminal domains from cholinesterase assemble with one proline-rich attachment domain (PRAD) in the secretory pathway. EMBO J. 1998, 17, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Allderdice, P.W.; Gardner, H.A.; Galutira, D.; Lockridge, O.; LaDu, B.N.; McAlpine, P.J. The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26. Genomics 1991, 11, 452–454. [Google Scholar] [CrossRef]
- Getman, D.K.; Eubanks, J.H.; Camp, S.; Evans, G.A.; Taylor, P. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7. Am. J. Hum. Genet. 1992, 51, 170–177. [Google Scholar] [PubMed]
- Krejci, E.; Thomine, S.; Boschetti, N.; Legay, C.; Sketelj, J.; Massoulie, J. The mammalian gene of acetylcholinesterase-associated collagen. J. Biol. Chem. 1997, 272, 22840–22847. [Google Scholar] [CrossRef]
- Perrier, A.L.; Massoulie, J.; Krejci, E. PRiMA: The membrane anchor of acetylcholinesterase in the brain. Neuron 2002, 33, 275–285. [Google Scholar] [CrossRef]
- Dvir, H.; Harel, M.; Bon, S.; Liu, W.Q.; Vidal, M.; Garbay, C.; Sussman, J.L.; Massoulie, J.; Silman, I. The synaptic acetylcholinesterase tetramer assembles around a polyproline II helix. EMBO J. 2004, 23, 4394–4405. [Google Scholar] [CrossRef]
- Larson, M.A.; Lockridge, O.; Hinrichs, S.H. Polyproline promotes tetramerization of recombinant human butyrylcholinesterase. Biochem. J. 2014, 462, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Duysen, E.G.; Snow, B.; Jensen, N.S.; Manne, V.; Lockridge, O.; Chilukuri, N. Gene-delivered butyrylcholinesterase is prophylactic against the toxicity of chemical warfare nerve agents and organophosphorus compounds. J. Pharmacol. Exp. Ther. 2011, 337, 92–101. [Google Scholar] [CrossRef]
- Boyko, K.M.; Baymukhametov, T.N.; Chesnokov, Y.M.; Hons, M.; Lushchekina, S.V.; Konarev, P.V.; Lipkin, A.V.; Vasiliev, A.L.; Masson, P.; Popov, V.O.; et al. 3D structure of the natural tetrameric form of human butyrylcholinesterase as revealed by cryoEM, SAXS and MD. Biochimie 2019, 156, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O.; Adkins, S.; La Du, B.N. Location of disulfide bonds within the sequence of human serum cholinesterase. J. Biol. Chem. 1987, 262, 12945–12952. [Google Scholar] [CrossRef]
- Schopfer, L.M.; Lockridge, O. Tetramer-organizing polyproline-rich peptides differ in CHO cell-expressed and plasma-derived human butyrylcholinesterase tetramers. Biochim. Biophys. Acta 2016, 1864, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Biberoglu, K.; Schopfer, L.M.; Tacal, O.; Lockridge, O. The proline-rich tetramerization peptides in equine serum butyrylcholinesterase. FEBS J. 2012, 279, 3844–3858. [Google Scholar] [CrossRef]
- Li, H.; Schopfer, L.M.; Masson, P.; Lockridge, O. Lamellipodin proline rich peptides associated with native plasma butyrylcholinesterase tetramers. Biochem. J. 2008, 411, 425–432. [Google Scholar] [CrossRef]
- Peng, H.; Schopfer, L.M.; Lockridge, O. Origin of polyproline-rich peptides in human butyrylcholinesterase tetramers. Chem. Biol. Interact. 2016, 259, 63–69. [Google Scholar] [CrossRef]
- Saxena, A.; Belinskaya, T.; Schopfer, L.M.; Lockridge, O. Tetramer organizing polyproline-rich peptides identified by mass spectrometry after release of the peptides from Hupresin-purified butyrylcholinesterase tetramers isolated from milk of domestic pig (Sus scrofa). Data Brief 2018, 20, 1607–1619. [Google Scholar] [CrossRef]
- Koomen, J.M.; Li, D.; Xiao, L.C.; Liu, T.C.; Coombes, K.R.; Abbruzzese, J.; Kobayashi, R. Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. J. Proteome Res. 2005, 4, 972–981. [Google Scholar] [CrossRef]
- Saxena, A.; Belinskaya, T.; Schopfer, L.M.; Lockridge, O. Characterization of butyrylcholinesterase from porcine milk. Arch. Biochem. Biophys. 2018, 652, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Biberoglu, K.; Schopfer, L.M.; Saxena, A.; Tacal, O.; Lockridge, O. Polyproline tetramer organizing peptides in fetal bovine serum acetylcholinesterase. Biochim. Biophys. Acta 2013, 1834, 745–753. [Google Scholar] [CrossRef]
- Chilukuri, N.; Duysen, E.G.; Parikh, K.; Sun, W.; Doctor, B.P.; Lockridge, O.; Saxena, A. Adenovirus-mediated gene transfer of human butyrylcholinesterase results in persistent high-level transgene expression in vivo. Chem. Biol. Interact. 2008, 175, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, L.M.; Delacour, H.; Masson, P.; Leroy, J.; Krejci, E.; Lockridge, O. The C5 Variant of the Butyrylcholinesterase Tetramer Includes a Noncovalently Bound 60 kDa Lamellipodin Fragment. Molecules 2017, 22, 1083. [Google Scholar] [CrossRef] [PubMed]
- Spieker, J.; Mudersbach, T.; Vogel-Hopker, A.; Layer, P.G. Endochondral Ossification Is Accelerated in Cholinesterase-Deficient Mice and in Avian Mesenchymal Micromass Cultures. PLOS ONE 2017, 12, e0170252. [Google Scholar] [CrossRef]


| Protein Donor | Swiss Prot Accession | Observed Peptide A | Pept# B | Spectral Count C | Prot D Match |
|---|---|---|---|---|---|
| Lamellipodin | Q70E73 | PSPPL PPPPP PPPPP PPPPP PPPPP LPSQ | 39 | 1937 | 1 |
| UDP-N-acetylglucosamine transferase and deubiquitinase ALG13 isoform 1 | Q9NP73 | LPPPP PPPPP PPPPP PPPPP P | 17 | 239 | 3 |
| Synaptopodin | Q8N3V7 | APPPP PPPPP PPP | 4 | 183 | 5 |
| Leiomodin-2 | Q6P5Q4 | LPPPP PPPPP P and TPPPP PPPPP PPPP | 2 4 | 180 | 1 |
| Acetylcholinesterase membrane anchor precursor PRiMA variant II | Q86XR5 | LPPPP PPPPP P | 2 | 121 | 27 |
| Formin-like protein 1 | O95466 | LPPPP PPPPP PP | 4 | 67 | 2 |
| Zinc finger protein ZIC5 | Q96T25 | SPPPP PPPPP PP and LPPPP PPPPP PPPPP P | 2 10 | 61 | 1 |
| Homeobox protein Hox-B4 | P17483 | GPPPP PPPPP PPP | 4 | 47 | 2 |
| Zinc finger CCCH domain-containing protein 4 | Q9UPT8 | GPPPP PPPPP PPP | 4 | 47 | 2 |
| Diaphanous 1 | O60610 | STTPP PPPPP PPPPP P | 5 | 38 | 1 |
| Zinc finger homeobox protein 4 | Q86UP3 | TPPPP PPPPP PPPPP PPPPP PSA | 10 | 23 | 1 |
| Protein piccolo | Q9Y6V0 | PPPPP PPPPP PPPPP PPPPP PL and QPPPP PPPPP PPPPP P | 11 5 | 17 | 1 |
| Formin-binding protein 4 | Q8N3X1 | EPPPP PPPPP PP | 2 | 36 | 3 |
| Protein Donor | Accession | Composite Peptide A | Pept# B | Spect C Count |
|---|---|---|---|---|
| Lysine-specific demethylase 6B | XP_005657086 | PLPPP PLPPP PPPPP PPPPP PPLPG LAT | 23 | 210 |
| Acrosin | P08001 | PAPPP APPPP PPPPP PPPPP PPPPP QQ | 25 | 138 |
| Proline-rich protein 12 | XP_003127395 | APPPP PPPPP PPPAS EPK and LPPPP PPPPP PPPPP PPPPP | 5 11 | 123 |
| Homeobox protein Hox-B4 | XP_003131596 | RDPGP PPPPP PPPPP PPPPG L | 11 | 116 |
| Proline-rich membrane anchor 1 | XP_003482358 | PPPPL PPPPP PPPPP R | 7 | 107 |
| Zinc finger homeobox protein 4 | XP_005663076 | TPPPP PPPPP PPPPP PPPPP SA and TPPPP PPPPP PPPPP SSL | 8 4 | 70 29 |
| Zinc finger CCCH domain -containing protein 4 | XP_005664683 | GGPPP PPPPP PPPPG PPQM | 4 | 33 |
| Disabled homolog 2-interacting protein-like isoform 1 | XP_003353684 | IDQPP PPPPP PPPAP R | 1 | 12 |
| FH2 domain-containing protein 1 | XP_005666867 | PPPPS PPPPP PPPP | 4 | 10 |
| WAS/WASL-interacting protein family member isoform X1 | NP_001231241 | MPIPP PPPPP PGPPP PPTF | 2 | 6 |
| Protein FAM171A2 | XP_005668832 | AAAPP PPPPP PPAPP R | 1 | 4 |
| Proline-rich protein 16 | XP_005655053 | PNPPP PPPR | 1 | 1 |
| Donor Protein | Accession Number |
|---|---|
| Lamellipodin | (EGW06139 Cricetulus griseus) |
| Zinc finger homeobox protein 4 | (ERE85184 Cricetulus griseus) |
| Leiomodin-2 | (ERE89074 Cricetulus griseus) |
| Homeobox protein Hox-B4 | (NP_034589 Mus musculus) |
| Zinc finger CCCH domain-containing protein 4 | (Q6ZPZ3 Mus musculus) |
| Donor Protein | Accession Number |
|---|---|
| Lamellipodin | Q70E73 (Homo sapiens) |
| Zinc finger homeobox protein 4 | NP_001180156 (Bos Taurus) |
| Leiomodin-2 | NP_001098857 (Bos Taurus) |
| UDP-N-acetyl glucosamine transferase ALG13 subunit homolog | NP_001093392 (Homo sapiens) |
| Protein Piccolo | Q9Y6V0 (Homo sapiens) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lockridge, O.; Schopfer, L.M. Polyproline-Rich Peptides Organize Four Cholinesterase Subunits into a Tetramer; BChE and AChE Scavenge Polyproline Peptides Released during Metabolic Turnover. Proceedings 2020, 62, 5. https://doi.org/10.3390/proceedings2020062005
Lockridge O, Schopfer LM. Polyproline-Rich Peptides Organize Four Cholinesterase Subunits into a Tetramer; BChE and AChE Scavenge Polyproline Peptides Released during Metabolic Turnover. Proceedings. 2020; 62(1):5. https://doi.org/10.3390/proceedings2020062005
Chicago/Turabian StyleLockridge, Oksana, and Lawrence M. Schopfer. 2020. "Polyproline-Rich Peptides Organize Four Cholinesterase Subunits into a Tetramer; BChE and AChE Scavenge Polyproline Peptides Released during Metabolic Turnover" Proceedings 62, no. 1: 5. https://doi.org/10.3390/proceedings2020062005
APA StyleLockridge, O., & Schopfer, L. M. (2020). Polyproline-Rich Peptides Organize Four Cholinesterase Subunits into a Tetramer; BChE and AChE Scavenge Polyproline Peptides Released during Metabolic Turnover. Proceedings, 62(1), 5. https://doi.org/10.3390/proceedings2020062005