Abstract
This study aimed to assess the effect of the primary phytochemicals from coffee by-products and two aqueous extracts from the coffee husk and silverskin on lipid and glucose metabolism regulation in hepatocytes using an in vitro model of non-alcoholic fatty liver disease. Coffee husk and silverskin were used to prepare two aqueous extracts (CHE and CSE, respectively) using water. The phytochemical composition was determined using UPLC-MS/MS analysis. HepG2 cells were co-treated with 10–50 µmol L‒1 of either pure caffeine, chlorogenic acid, caffeic acid, protocatechuic acid, or gallic acid, and kaempferol, CHE, or CSE (20–100 µg mL‒1) in the presence or absence of palmitic acid (PA, 500 µmol L‒1). Different biomarkers of cell metabolism were assessed 24 h after the co-treatment in cell supernatants and lysates using chemical, biochemical, and immunochemical techniques. Phytochemicals from coffee by-products decreased PA-triggered lipid accumulation (16–94%, p < 0.05) by reducing fatty acid synthase activity and stimulating lipolysis (8–83%, p < 0.05). CHE, CSE, and therein-bioactive compounds promoted glucose uptake (13–45%) via the increase in the phosphorylation of the insulin receptor (1.9- to 2.7-fold), protein kinase B (AKT) (1.4- to 3.1-fold), AMPKα (1.6- to 2.4-fold), and PTEN (2.0- to 4.2-fold). In conclusion, our results proved that phytochemicals from coffee by-products, mainly caffeine and chlorogenic acid, could regulate hepatic lipid and glucose metabolism. Overall, our results generate new insights into the use of coffee by-products as a sustainable food ingredient to encounter non-alcoholic fatty liver disease.
1. Introduction
The World Health Organization pinpoints chronic diseases as being responsible for 71% of all deaths worldwide [1]. Metabolic disorders are caused by defective cellular metabolic processes triggering energy and redox imbalances and the induction of many organism pathophysiological conditions. Chronic diseases such as obesity and type II diabetes lead to non-alcoholic fatty liver disease (NAFLD). The global prevalence of NAFLD is currently estimated to be 24% [2]. These diseases could be prevented through nutrition and adequate food patterns [3].
Coffee by-products, including coffee husk and silverskin, are considered a source of bioactive compounds. Owing to their proven ability to regulate cellular metabolic processes, phytochemicals from coffee by-products could modulate liver metabolism, preventing NAFLD. They are generated in large amounts during coffee harvesting and processing and generally settled in the environment, causing ecological problems [4]. They contain considerable amounts of antioxidant compounds, including caffeine and phenolics, that could be recovered for their use as value-added products [5,6]. In this sense, coffee by-products have been recently verified as safe food ingredients [7]. Phytochemicals from coffee by-products, primarily caffeine and chlorogenic acid, have demonstrated in vitro and in vivo potential to alleviate the symptoms of type 2 diabetes mellitus by increasing insulin secretion by β-cells and protecting the pancreas from oxidative stress [8,9]. The effects of these by-products and the bioactive compounds present therein have not been investigated to date. Hence, this work’s goal was to evaluate the impact of the primary phytochemicals from coffee by-products and two aqueous extracts from the coffee husk and silverskin on lipid regulation and glucose metabolism in hepatocytes using an in vitro model of non-alcoholic fatty liver disease.
2. Materials and Methods
2.1. Materials
Minimum essential medium (MEM) was purchased from Corning Cellgro (Manassas, VA, USA), and fetal bovine serum (FBS), penicillin-streptomycin (100×), and 0.25% trypsin-EDTA were obtained from Gibco Life Technologies (Grand Island, NY, USA). Pure bioactive compounds (purity ≥ 96%), including caffeine, chlorogenic acid, caffeic acid, protocatechuic acid, gallic acid, and kaempferol, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Extrasynthese (Genay, France).
2.2. Aqueous Extraction of Phytochemicals from Coffee By-Products and UPLC-MS/MS Characterization
Coffee silverskin from Colombia was provided by Fortaleza S.A. (Spain) and coffee husk by “Las Morenitas” (Nicaragua), both from the Arabica species. Based on validated extraction protocols [10], a phenolic aqueous extract from each coffee by-product was prepared. After milling and sieving, ground coffee silverskin (25 g) was added to 500 mL of boiling water (100 °C) and stirred for 10 min. Coffee husk (10 g) was added to boiling water (500 mL) and stirred for 90 min. Coffee silverskin aqueous extract (CSE) and coffee husk aqueous extract (CHE) were filtered and frozen at −20 °C for 24 h. Extracts were freeze-dried and stored at −20 °C until further use. The analysis of targeted phytochemicals was carried out by UPLC-ESI–MS/MS following a method previously described [11].
2.3. Cell Culture
The HepG2 human hepatocyte cell line obtained from the American Type Culture Collection (Manassas, VA, USA) was grown at 37 °C in an atmosphere of 5% CO2. HepG2 cells were cultured in MEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% sodium pyruvate. Cells were plated at a density of 105 cells cm−2 flask.
2.4. Experimental Design
Hepatocytes were treated with pure phytochemicals (caffeine, chlorogenic acid, caffeic acid, protocatechuic acid, gallic acid, and kaempferol) from coffee by-products (10, 20, or 50 µmol L−1) or the aqueous extracts (20, 50, or 100 µg mL−1) in the presence or absence of palmitic acid (PA, 500 µmol L−1) for 24 h. Supernatants were collected and stored at –80 °C until further analysis. Cells were washed twice with ice-cold phosphate-buffered saline (PBS), lysed using the RIPA Lysis Buffer System (Santa Cruz Biotechnology, CA, USA) and centrifuged at 10,000× g, at 4 °C for 10 min to eliminate cell debris to be stored at –80 °C.
2.5. Cell Viability
Cell viability of cells treated with pure phytochemicals from coffee by-products (10, 20, or 50 µmol L−1) or the aqueous extracts (20, 50, or 100 µg mL−1) in the presence or absence of palmitic acid (500 µmol L−1) for 24 h was performed with the CellTiter 96 Aqueous One Solution Proliferation assay (Promega Corporation, Madison, WI, USA) following the manufacturer’s instructions.
2.6. Evaluation of the Effect of Phytochemicals from Coffee By-Products on Hepatic Lipid Metabolism
2.6.1. Determination of Cellular Lipid Accumulation
Oil Red O lipid staining was performed as previously described [12]. HepG2 cells were seeded in 24-well plates and treated with 500 µmol L−1 for 24 h. Pure phytochemicals (10, 20, or 50 µmol L−1) or extracts (20, 50, or 100 µg mL−1) were added to the culture media, and lipid staining was performed after 24 h. Results were expressed as fold change of lipid accumulation compared to the non-treated control before standardization with cell viability.
2.6.2. Lipolysis Assessment
The culture media was collected from palmitic acid-treated HepG2 cells after the 24 h co-treatment with palmitic acid (500 µmol L−1), pure phytochemicals (10, 20, or 50 µmol L−1), or the aqueous extracts (20, 50, or 100 µg mL−1) was tested for glycerol quantification using a glycerol cell-based assay kit (Cayman Chemical Item No. 10011725), and the cell lysates were assayed for intracellular triglycerides (TAG) using a TAG colorimetric assay kit (Cayman Chemical Item No. 10010303). Lipase activity was determined using a lipase activity assay kit (Cayman Chemical Item No. 700640).
2.6.3. Fatty Acid Synthase (FASN) Activity
FASN activity inhibition from HepG2 cells was determined through NADPH oxidation, as previously described [12]. Cell lysates were added to the reaction mixture that contained 1 mmol L−1 EDTA, 1 mmol L−1 dithiothreitol, 30 μmol L−1 acetyl-CoA, and 0.15 mmol L−1 NADPH in a final volume of 300 μL. The reaction was monitored at 340 nm for 3 min to determine background NADPH oxidation. The reaction was initiated by adding 50 μmol L−1 malonyl-CoA, and the decrease of absorbance at 340 nm was monitored for 20 min. The results of the FASN activity were expressed as μmol/min/mL.
2.6.4. Carnitine Palmitoyltransferase I (CPT-1) Activity
CPT-1 activity was measured as previously described [13]. To determine CPT-1 activity, protein from crude enzyme extract (10 μg) was assayed in 200 μL reaction buffer containing 100 mM Tris-HCl (pH 7.4), 0.12 mmol L−1 DTNB, 1.3 mg mL−1 BSA, 0.09% TritonX-100, and 75 μmol L−1 palmitoyl-CoA at pH 7.4. The reaction was initiated by adding 75 μmol L−1 L-carnitine and read at 412 nm after 3 min incubation at 37 °C. CPT-2 was extracted from crude enzyme extract by treating with Tween-20 (3 μL of detergent per mg protein) for 40 min at 0 °C. Tween-20 solubilizes CPT-2 but not CPT-1.
2.7. Evaluation of the Effect of Phytochemicals from Coffee By-Products on Hepatic Glucose Metabolism
2.7.1. Glucose Uptake Measurement
The effect of phytochemicals from coffee by-products on the glucose uptake was measured through 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose (2-NBDG) uptake, which was carried out as previously described [12]. HepG2 cells were cultured in a black 96-well plate with clear bottom and treated with palmitic acid (500 µmol L−1) and pure phytochemicals (10, 20, or 50 µmol L−1) or the aqueous extracts (20, 50, or 100 µg mL−1). Following a 24 h treatment, the cells were incubated for 2 h with glucose-free DMEM with 100 µmol L−1 2-NBDG. Then the cells were washed with PBS and the fluorescence was detected at an excitation/emission wavelength of 485 nm/535 nm.
2.7.2. Glucokinase (GK) Activity
GK activity was assessed using the method of Dhanesha et al. [14]. The cells were homogenized in a buffer containing 20 mmol L−1 K2HPO4, 1 mmol L−1 EDTA, 110 mmol L−1 KCl, and 5 mmol L−1 dithiothreitol (DTT) (pH 7.4). The homogenates were lysed through sonication using 3 bursts of 3 s each. The lysates were cleared at 12,000× g for 20 min at 4 °C. A 100 U mL−1 of glucose-6 phosphate dehydrogenase enzyme solution was added to a reaction cocktail containing glucose, 60 mmol L−1 Tris-HCl buffer (pH 9), 20 mmol L−1 MgCl2, 4 mmol L−1 ATP, and 0.9 mmol L−1 NADP. The reaction was equilibrated for 5 min, and 20 μg of protein mL−1 of the cell lysates were added. The absorbance was monitored at 320 nm in kinetic mode for 10 min. Correction of hexokinase activity was assessed by subtracting the activity measured with 0.5 mmol L−1 glucose (measures only low Km hexokinases) from the activity obtained at 360 mmol L−1 glucose (measures all hexokinases, including GK).
2.7.3. Gluconeogenesis Evaluation
HepG2 cells were seeded in 24-well plates and treated with 500 µmol L−1 for 24 h. Pure phytochemicals (10, 20, or 50 µmol L−1) or extracts (20, 50, or 100 µg mL−1) were added to the culture media. Then, HepG2 culture media were changed to glucose production buffer (glucose-free DMEM supplemented with 20 mmol L−1 sodium lactate and 2 mmol L−1 sodium pyruvate) and incubated for 4 h. Subsequently, media were collected to detect D-glucose using an Amplex Red Glucose/Glucose Oxidase Assay Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
2.7.4. Phosphoenolpyruvate Carboxykinase (PEPCK) Activity
PEPCK was measured using the NaHCO3 fixation assay as previously described [15]. Briefly, after removing the culture medium, each well (105 cells per well) was scraped into 500 μL of reaction buffer containing 150 μmol Tris/acetate (pH 7.2), 5 μmol sodium inosine-diphosphate, 10 μmol MnCl2, 250 μmol KCl, 10 mmol L−1 DTT, 2 mM glutathione, and 400 μmol KHCO3. The reaction began by the addition of 10 μL of 0.4 mol L−1 phosphoenolpyruvate and the process was terminated after 10 min of incubation at 25 °C by the addition of 1 mL of 6 N HCl and by placing the tube on ice. The reaction mixture for PEPCK contained 100 μmol of imidazole-HCl buffer, pH 6.6, 2 μmol of MnCl2, 1 μmol of GSH, 1.25 μmol of sodium IDP, 50 μmol L−1 of KHCO3, 2.5 μmol of NADH, 1.5 μmol of PEP, and 2 U of malate dehydrogenase. The final pH was 7.1. The optical density was then measured at 340 nm.
2.8. Evaluation of the Effect of Phytochemicals from Coffee By-Products on the Phosphorylation Pattern of Metabolism-Related Proteins
HepG2 hepatocytes were cultured and treated with palmitic acid (500 µmol L−1) in the presence/absence of CSE or CHE (200 µg mL−1) for 24 h. After treatment, the cells were serum-starved for 30 min, followed by 10 min stimulation with 10 ng mL−1 of insulin. Cell lysates were applied following the manufacturer’s instructions to the insulin receptor and AKT signaling pathway microarrays (RayBiotech, AAH-INSR and AAH-AKT). The array signal was visualized on a GelLogic 4000 Pro Imaging System (Carestream Health, Inc., Rochester, NY, USA).
2.9. Bioinformatic Analysis
The resulting differentially phosphorylated proteins and protein–protein interactions were searched using Metascape (https://metascape.org/) [16]. The differentially expressed proteins were categorized based on the biological process and molecular function and further analyzed for KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis by using the KEGG database (http://www.genome.jp/kegg/kaas/). Circos plot, enrichment analysis, and protein–protein interacting network of the studied proteins and their nearest functional and predicted associations were established.
2.10. Statistical Analysis
Sample preparation and determinations were performed in triplicate. Results are expressed as the mean ± standard deviation (SD) (n = 3) and analyzed by one-way analysis of variance (ANOVA) and post hoc Tukey test. Differences were considered significant at p < 0.05. The statistical analysis of the results was performed using SPSS 24.0. Multivariate analyses were carried out with XLSTAT 2018 for Microsoft Excel 2016.
3. Results
3.1. Coffee Silverskin and Coffee Husk Were Mainly Composed of Caffeine and Chlorogenic Acid
The UPLC-ESI-MS/MS phytochemical profile of CSE and CHE showed that chlorogenic acid (2.8 mg g−1) and caffeic acid (0.5 mg g−1) were the main phenolics in CSE, representing 97% of the total phenolic compounds measured. In CHE, the major one was chlorogenic acid (3.5 mg g−1), but protocatechuic acid, kaempferol-3-O-galactoside, and gallic acid were also present, accounting for 94% of the total amount of studied phenolics, with caffeic acid appearing in a less significant concentration. Hence, the main bioactive compounds found among CSE and CHE were selected as pure compounds for subsequent analyses. Caffeine was the primary alkaloid found in CSE and CHE, being in higher concentration in the husk than in the silverskin (19.2 vs. 9.8 mg mL−1). None of the treatments, neither CSE, CHE (20, 50, or 100 µg mL−1), nor pure compounds (10, 20, or 50 µmol L−1), exerted cytotoxicity in HepG2 hepatocytes at the concentrations tested (p > 0.05).
3.2. Phytochemicals from Coffee By-Products Inhibited de Novo Lipogenesis and Stimulated Fatty Acid Oxidation
Phytochemicals from coffee by-products and the two aqueous extracts decreased PA-triggered lipid accumulation (16–94%, p < 0.05). Caffeine and chlorogenic acid showed a higher (p < 0.05) potential among the pure phytochemicals. Concurrently, coffee by-product aqueous extracts and the phytochemicals present therein could reduce the content of intracellular TAG. An increase (p < 0.05) in the lipolysis rate (8–83%) was observed by evaluating the activity of lipase and quantifying the content of released glycerol. On the other hand, de novo lipogenesis was attenuated as indicated by the diminished (p < 0.05) FASN activity (14–37%). Caffeine, chlorogenic acid, and protocatechuic acid exhibited the most significant effects. Regarding the effects on fatty acid β-oxidation, CSE, CHE, and most phytochemicals showed induction in the activity of CPT-1. The bioactive compounds from coffee by-products could then modulate hepatic lipid metabolism via inhibition of de novo lipogenesis and activation of fatty acid mobilization and β-oxidation.
3.3. Bioactive Compounds from Coffee By-Products Promoted Glucose Uptake and Diminished Gluconeogenesis
CHE, CSE, and therein-bioactive compounds significantly (p < 0.05) promoted glucose uptake (13–45%) in both basal conditions and after PA stimulation. Besides, the bioactive compounds from coffee by-products stimulated GK activity (17–63%, p < 0.05), indicating that absorbed glucose was mobilized to be metabolized during glycolysis. Protocatechuic and chlorogenic acids exhibited the most significant effects, acting as insulin-like treatments. Additionally, CSE and CHE reduced gluconeogenesis by 23–56% (p < 0.05). All the phytochemicals exhibited similar results as the aqueous extracts from coffee by-products. The main enzyme regulating gluconeogenesis, PEPCK, suffered reductions in HepG2 cell activity co-treated with PA and both pure phytochemicals and the extracts from coffee by-products (9–41%, p < 0.05). Therefore, the phytochemicals present in coffee by-products regulated glucose metabolism by promoting glucose uptake and diminishing gluconeogenesis.
3.4. Modulations of Lipid and Glucose Metabolism Were Governed via Insulin, PI3K-AKT, and mTOR Pathways
To better understand the mechanism guiding the regulations previously observed on lipid and glucose metabolism, the phosphorylation pattern of multiple proteins associated with cell metabolism was studied. CSE and CHE modulated the phosphorylation of 26 out of 30 studied proteins. CSE modulated 23/30 whereas CHE only 21 (Figure 1A). The most significant changes were those of the insulin receptor (1.9- to 2.7-fold), protein kinase B (AKT) (1.4- to 3.1-fold), AMPKα (1.6- to 2.4-fold), and PTEN (2.0- to 4.2-fold). Nonetheless, other important proteins were also regulated, including mTOR, LKB1, IRS-1, and p53. Most biological processes controlled by CSE were also altered
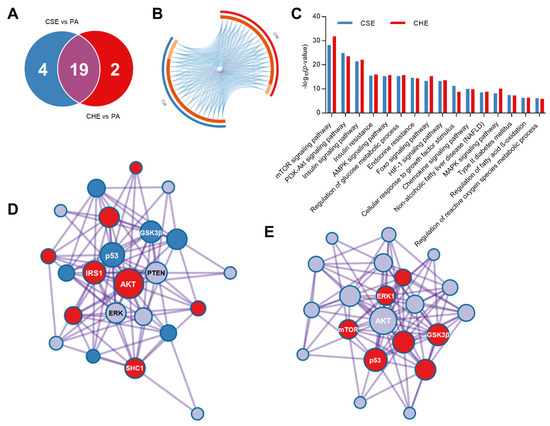
Figure 1.
Venn diagram illustrating the overlap of differentially phosphorylated protein in adipocytes by coffee silverskin (CSE) and coffee husk (CHE) aqueous phenolic extracts (A), circos plot illustrating the biological processes modulated by CSE and CHE indicating which are shared among treatments (B), KEGG pathways associated with the differentially phosphorylated proteins (C), and protein–protein interaction networks built with Metascape from the differentially phosphorylated protein in hepatocytes by CSE (D) and CHE (E).
By CHE (Figure 1B,C), protein–protein interaction corroborated the relationship established among proteins from the same pathway (Figure 1D,E). CSE up-phosphorylated proteins were clustered in two groups (AKT and mTOR pathways) where only one cluster was observed for the up-phosphorylated proteins of CHE (mTOR signaling pathway).
4. Discussion
The global obesity epidemic is accompanied by an increasing prevalence of associated metabolic disorders [17]. For the first time, we present the impact of two well-characterized phenolic-rich extracts from coffee by-products and five pure phenolic compounds, composing them on an in vitro model of NAFLD. NAFLD is characterized by a dysregulated hepatic metabolism conducting to insulin resistance, lipid accumulation, and further inflammation and oxidative stress [18]. The use of food-based strategies to prevent metabolic diseases has been previously evidenced. We previously evidenced the modulatory activity of bioactive compounds from coffee by-products on obesity-related inflammation in adipocytes and the alleviation of mitochondrial dysfunction and insulin resistance [19].
The activation of insulin, AKT, and mTOR signaling pathways, among other signaling cascades, demonstrated that the phytochemicals from coffee by-products could regulate cell metabolism. Regulating insulin and PI3K-AKT pathways improved HepG2 insulin sensitivity, favoring catabolic pathways, including lipid mobilization (lipolysis) and oxidation, glucose uptake and glycolysis, and diminished anabolic pathways (lipogenesis and gluconeogenesis) [20]. Recent studies proved the effect of food phytochemicals on reducing HepG2 PA-triggered lipid accumulation. Activating AMPK signaling led to reduced FASN activity and increased CPT-1 activity [21,22]. Previous research also demonstrated how phytochemicals promoting INSR, AKT, GSK3β, and AMPK phosphorylation and activation can also promote glucose uptake and reduce gluconeogenesis via reducing PEPCK protein expression and activity [23]. Therefore, the observed effects validate the regulatory role of phytochemicals from coffee silverskin and coffee husk. The regulation of cell metabolic signaling leads to a more functional phenotype in HepG2 cells than PA-treated control. Despite the potential biological activity of phytochemicals from coffee by-products, phenolic compound bioefficacy is conditioned by their low bioavailability. These phytochemicals are only partially absorbed in the gastrointestinal tract. Phytochemicals’ effect could be somewhat altered after being metabolized by the microbiota or in the same liver (methylation, sulfation, and glucuronidation). Future animal and clinical investigations will be necessary to confirm the effects observed in vitro and determine the absorption and metabolism of coffee silverskin and husk phytochemicals and their beneficial and potentially harmful effects.
5. Conclusions
In conclusion, our results proved that phytochemicals from coffee by-products, mainly caffeine and chlorogenic acid, could regulate hepatic lipid and glucose metabolism. Furthermore, we provide new knowledge on the underlying mechanism of action of the bioactive compounds composing coffee silverskin and coffee husk. Overall, our results generate new insights into the use of coffee by-products as a sustainable food ingredient to encounter NAFLD.
Author Contributions
Conceptualization, M.R.-H. and E.G.d.M.; formal analysis, M.R.-H.; investigation, M.R.-H.; data curation, M.R.-H.; writing—original draft preparation, M.R.-H.; writing—review and editing, M.R.-H. Y.A., M.A.M.-C., and E.G.d.M.; visualization, M.R.-H. and Y.A.; supervision, Y.A., M.A.M.-C., and E.G.d.M.; funding acquisition, Y.A., M.A.M.-C., and E.G.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA–NIFA–HATCH project to E.G.D.M. (1014457) and by the COCARDIOLAC project from the Spanish Ministry of Science and Innovation (RTI 2018-097504-B-I00). M. Rebollo-Hernanz received funding from the FPU program of the Ministry of Science, Innovation, and Universities for his predoctoral fellowship (FPU15/04238) and the support for the international research stays at the University of Illinois, Urbana–Champaign (EST17/00823, EST18/0064).
Acknowledgments
The authors thank Lindsey Willis’ help for cell lysate preparation and protein quantification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 May 2019).
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Dorsey, B.M.; Jones, M.A. Chapter 2—Healthy components of coffee processing by-products. In Handbook of Coffee Processing By-Products; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 27–62. ISBN 9780128112908. [Google Scholar]
- Del Castillo, M.D.; Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Martín-Cabrejas, M.A.; Farah, A. CHAPTER 2. Coffee Antioxidants in Chronic Diseases. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 20–56. [Google Scholar]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.G.P.; San Andres, M.I.M.I.; Sanchez-Fortun, S.; del Castillo, M.D.M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Ramos, S.; Goya, L.; Mesa, M.D.; del Castillo, M.D.; Martín, M.Á. Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 2016, 89, 1015–1022. [Google Scholar] [CrossRef]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-α-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017, 61, 1700362. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Liu, Y.C.; Chang, C.J.; Pan, M.H.; Lee, M.F.; Pan, B.S. Hepatoprotective mechanism of freshwater clam extract alleviates non-alcoholic fatty liver disease: Elucidated: In vitro and in vivo models. Food Funct. 2018, 9, 6315–6325. [Google Scholar] [CrossRef] [PubMed]
- Dhanesha, N.; Joharapurkar, A.; Shah, G.; Dhote, V.; Kshirsagar, S.; Bahekar, R.; Jain, M. Exendin-4 reduces glycemia by increasing liver glucokinase activity: An insulin independent effect. Pharmacol. Rep. 2012, 64, 140–149. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L.; Song, H. The potential beneficial effects of phenolic compounds isolated from: A. pilosa Ledeb on insulin-resistant hepatic HepG2 cells. Food Funct. 2016, 7, 4400–4409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kong, X.; Yuan, H.; Guan, H.; Li, Y.; Niu, Y. Mangiferin improved palmitate-induced-insulin resistance by promoting free fatty acid metabolism in HepG2 and C2C12 cells via PPARα: Mangiferin improved insulin resistance. J. Diabetes Res. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Fan, L.; Chen, J.; Huang, R.; Qin, H. Improvement of Lipid and Glucose Metabolism by Capsiate in Palmitic Acid-Treated HepG2 Cells via Activation of the AMPK/SIRT1 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Pignitter, M.; Hochkogler, C.M.; Rohm, B.; Walker, J.; Bytof, G.; Lantz, I.; Somoza, V. Caffeine dose-dependently induces thermogenesis but restores ATP in HepG2 cells in culture. In Food and Function; Royal Society of Chemistry: London, UK, 2012; Volume 3, pp. 955–964. [Google Scholar]
- Cordero-Herrera, I.; Martín, M.Á.; Goya, L.; Ramos, S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).