Studying the Reaction of Peroxynitrite with Myoglobin for Meat Extract Samples Using Cobalt Phthalocyanine-Modified Screen-Printed Carbon Electrodes and a Flow Injection Analysis System †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
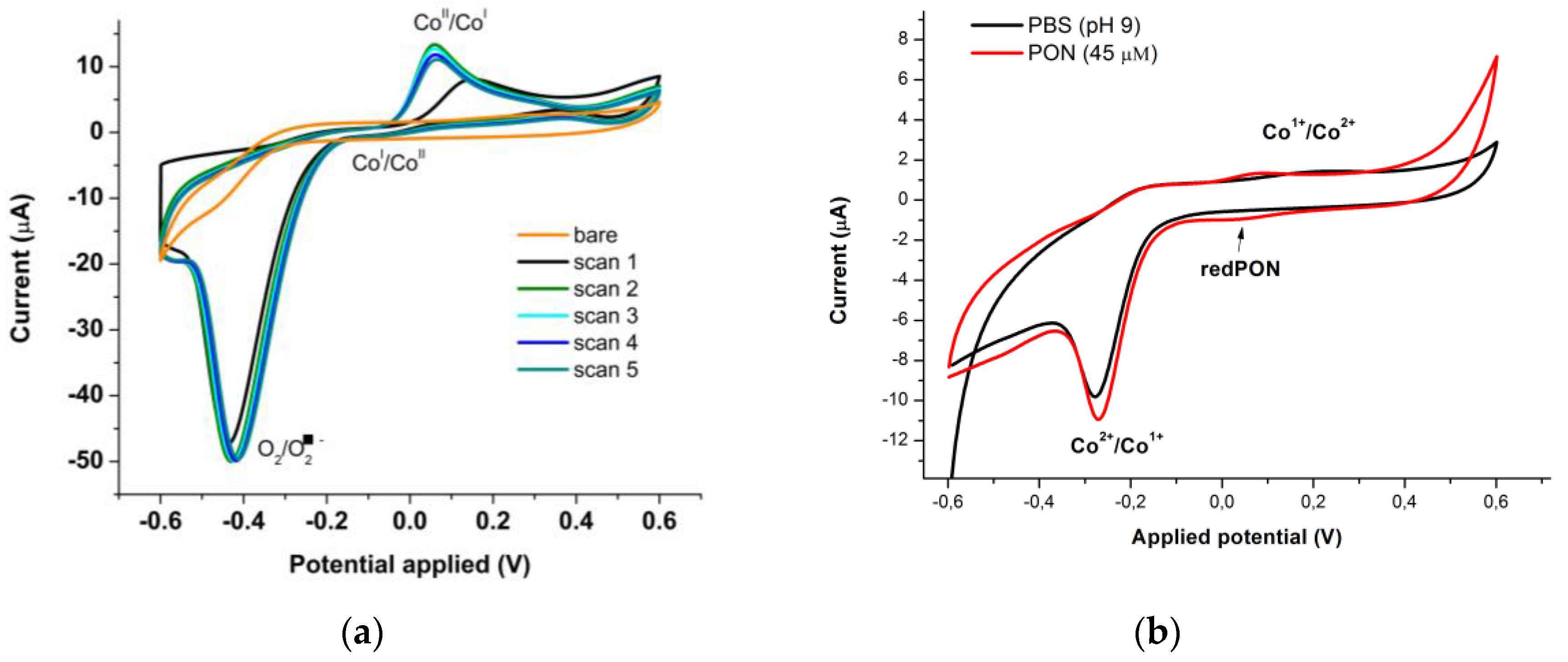
3.1. Batch Determination of Peroxynitrite Using SPCE/CoPc Electrodes
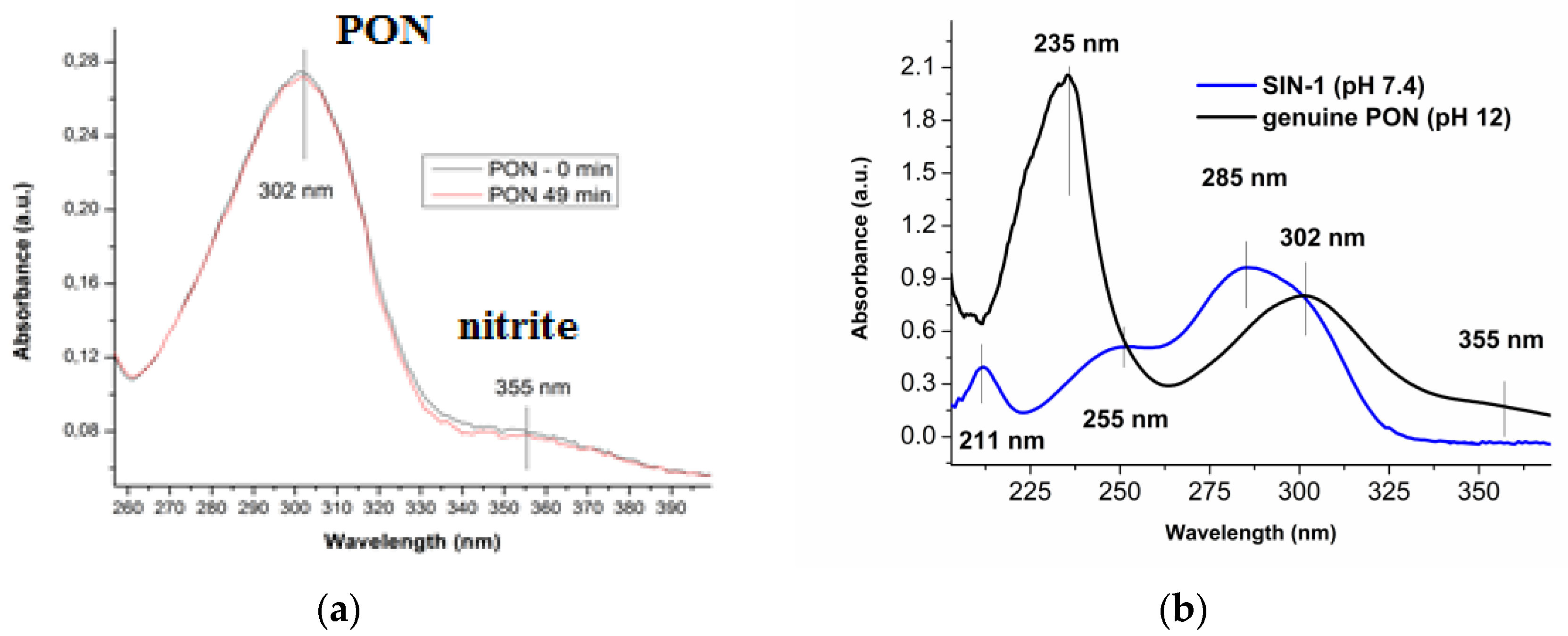
3.1.1. UV-Vis Determination of Synthesized PON and SIN-1
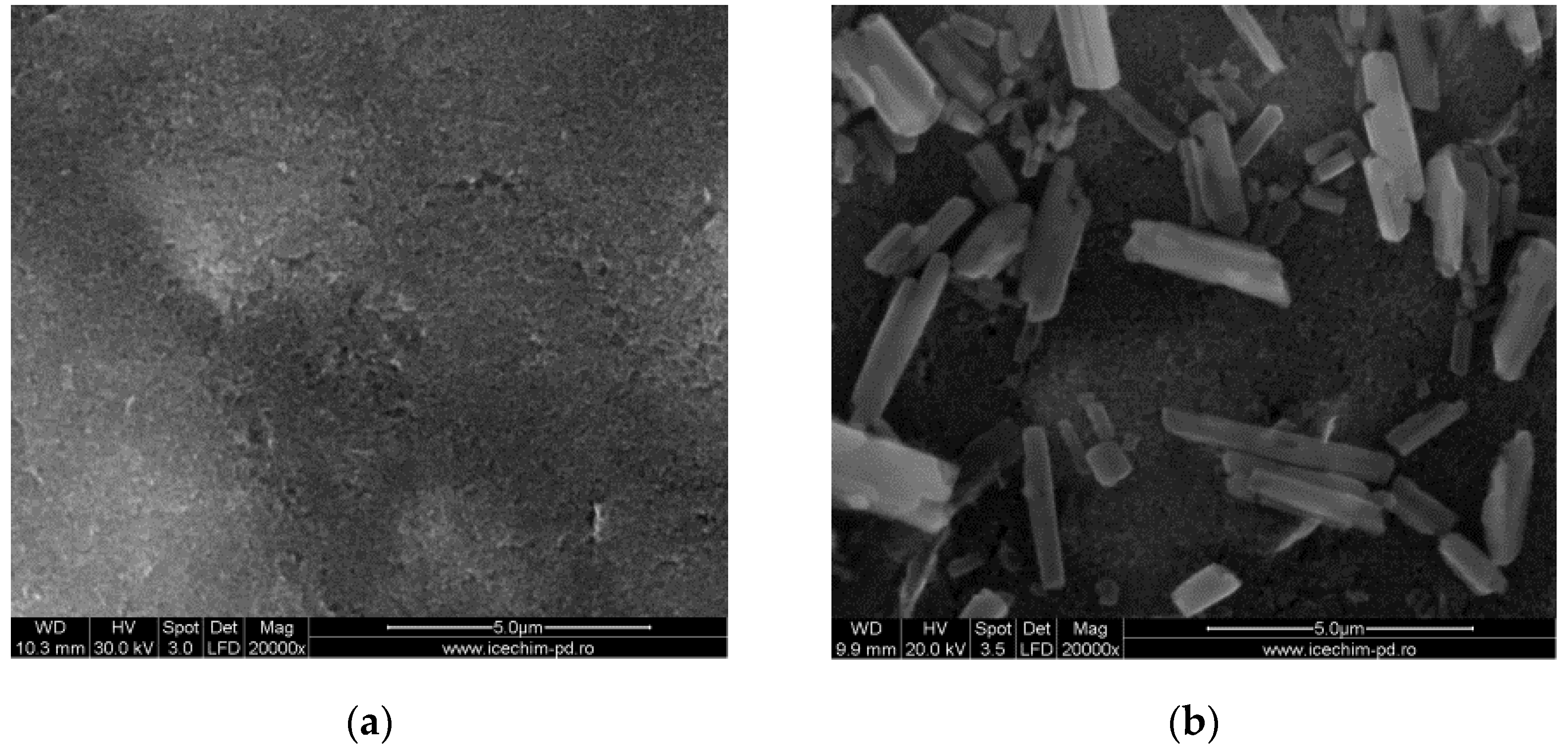
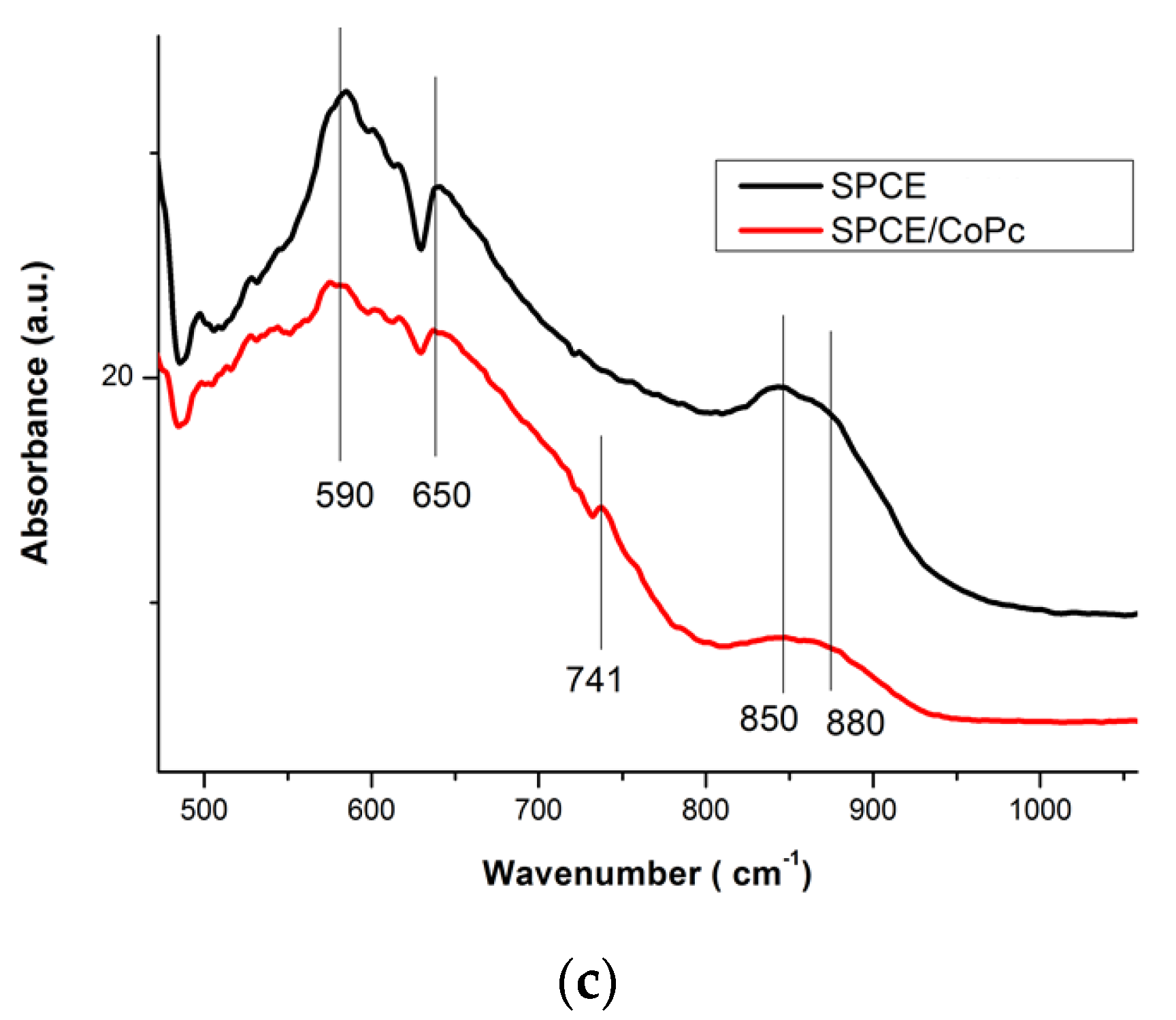
3.1.2. Characterization of the Deposited CoPc Films on the SPCE
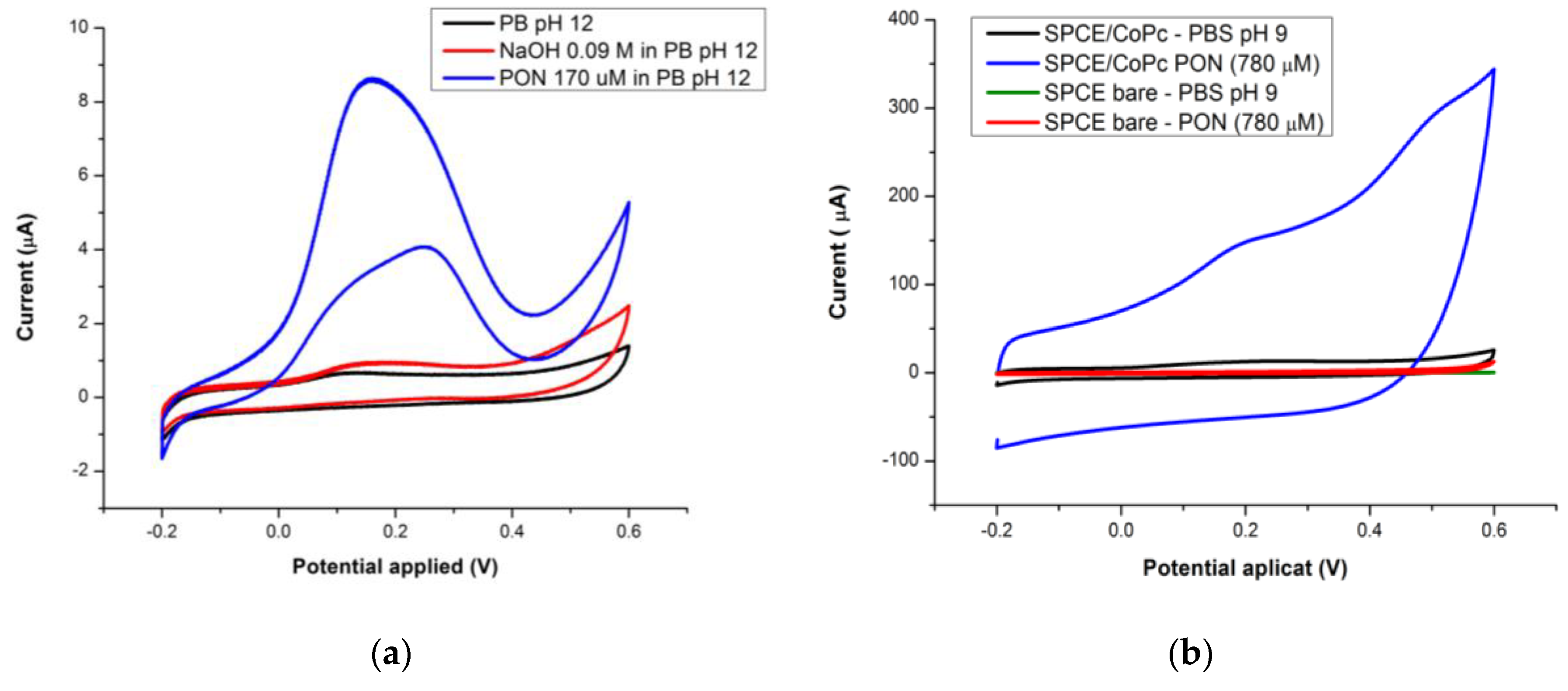
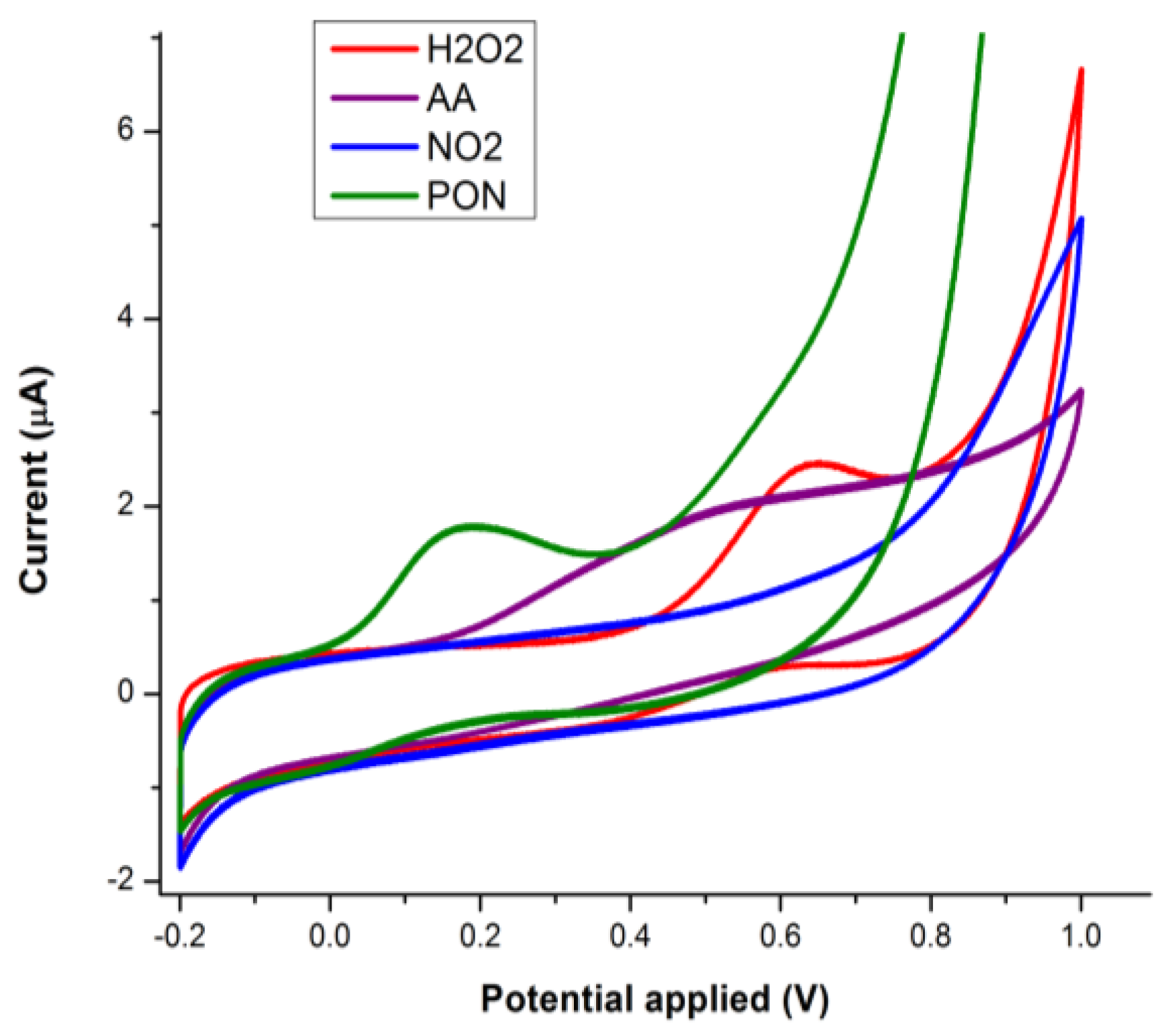
3.1.3. Batch Optimization of the CoPc Modified Electrodes for PON Detection
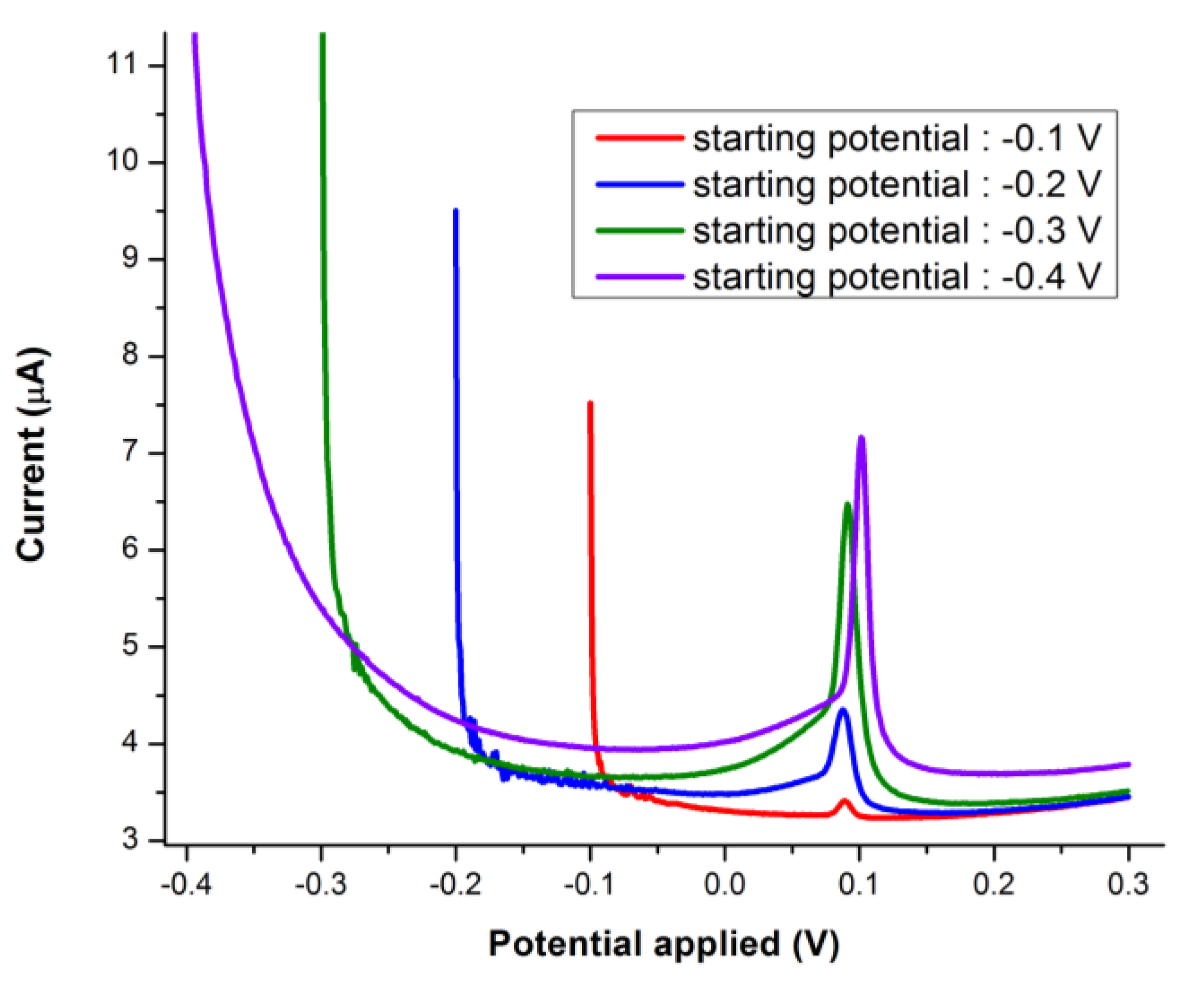
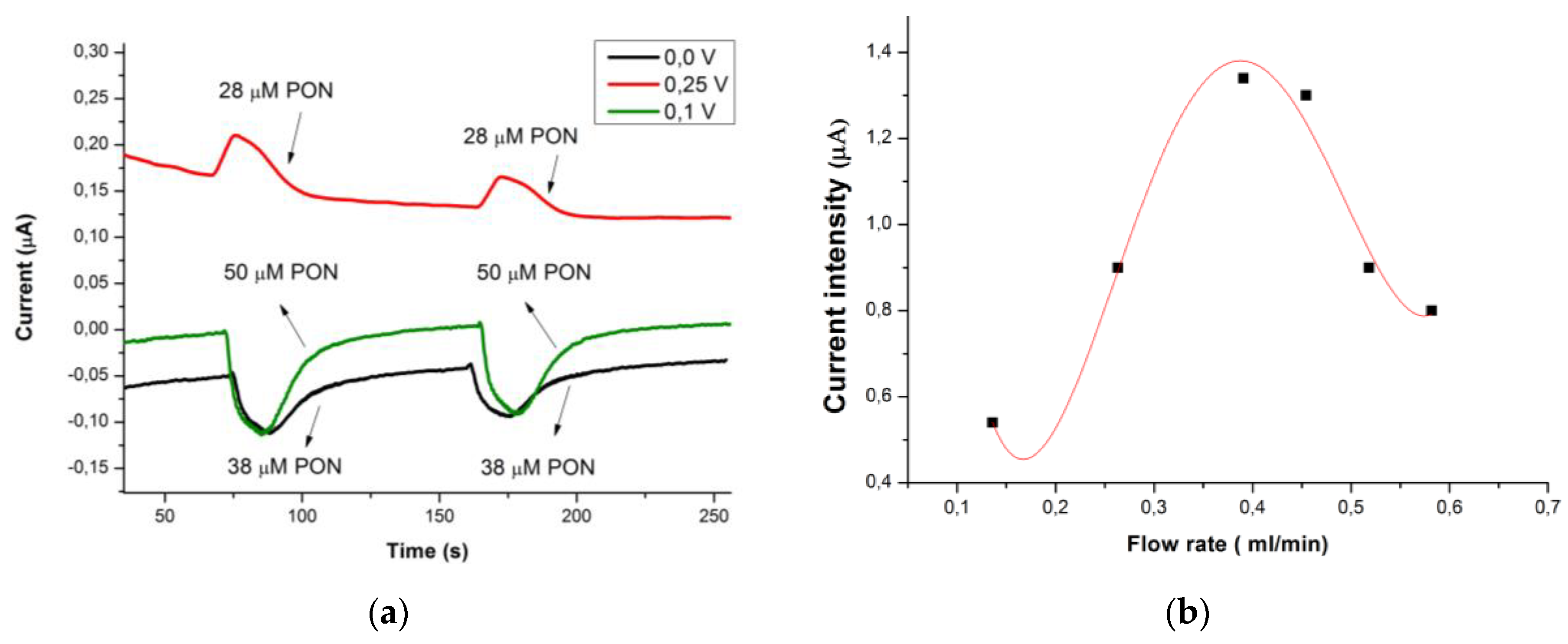
3.2. FIA Optimization of the SPCE/CoPc Electrodes for PON Detection
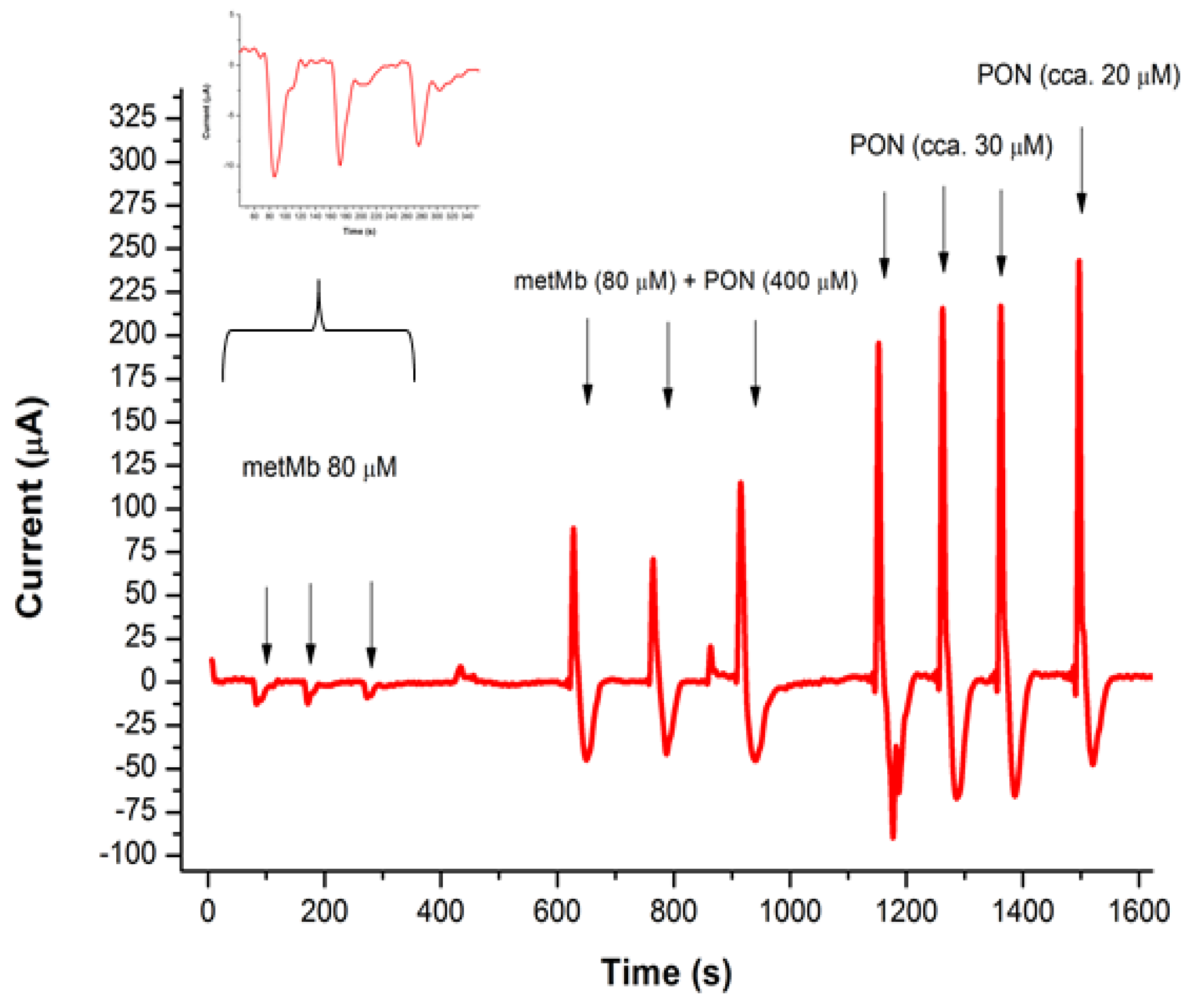
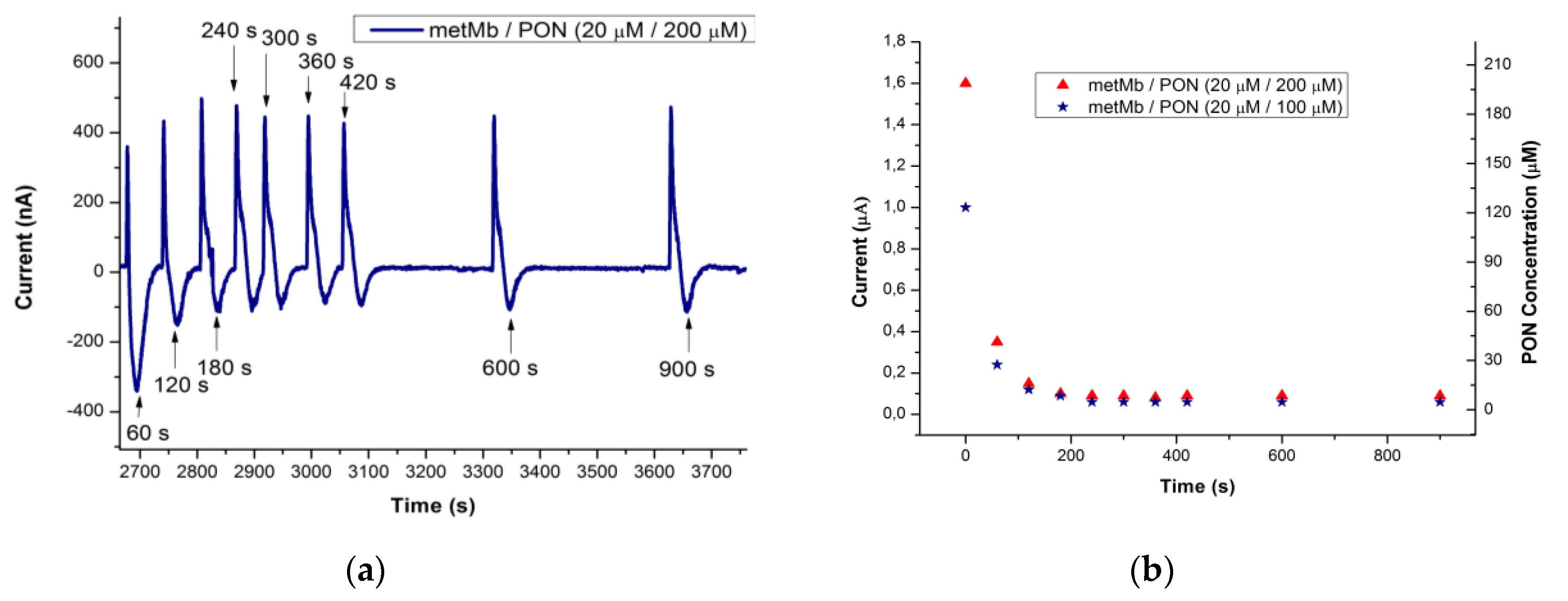
3.3. Studying the Reaction of Myoglobin in Buffered Solution and Peroxynitrite, Using FIA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Exner, M.; Herold, S. Kinetic and mechanistic studies of the peroxynitrite-mediated oxidation of oxymyoglobin and oxyhemoglobin. Chem. Res. Toxicol. 2000, 13, 287–293. [Google Scholar] [CrossRef]
- Vasilescu, A.; Vezeanu, A.; Liu, Y.; Hosu, I.S.; Worden, R.M.; Peteu, S.F. Meat freshness: Peroxynitrite’s oxidative role, its natural scavengers, and new measuring tools. In Instrumental Methods for the Analysis and Identification of Bioactive Molecules; Jayprakasha, G.K., Patil, B.S., Pellati, F., Eds.; ACS Symposium Series. 1185; American Chemical Society: Washington, DC, USA, 2014; pp. 303–332. [Google Scholar]
- Carlsen, C.U.; Møller, J.K.; Skibsted, L.H. Heme-iron in lipid oxidation. Co-ord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Kubant, R.; Malinski, C.; Burewicz, A.; Malinski, T. Peroxynitrite/Nitric Oxide Balance in Ischemia/Reperfusion Injury-Nanomedical Approach. Electroanalysis 2006, 18, 410–416. [Google Scholar] [CrossRef]
- Connolly, B.J.; Brannan, R.G.; Decker, E.A. Potential of Peroxynitrite to Alter the Color of Myoglobin in Muscle Foods. J. Agric. Food Chem. 2002, 50, 5220–5223. [Google Scholar] [CrossRef]
- Connolly, B.J.; Decker, E.A. Peroxynitrite induced discoloration of muscle foods. Meat Sci. 2004, 66, 499–505. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Kim, G.-D.; Yang, H.-S.; Joo, S.-T. Pigments and Color of Muscle Foods. In Methods in Food Analysis; Informa UK Limited: London, UK, 2014; pp. 44–61. [Google Scholar]
- Li, M.; Gong, X.; Li, H.-W.; Han, H.; Shuang, S.; Song, S.; Du, F. A fast detection of peroxynitrite in living cells. Anal. Chim. Acta 2020, 1106, 96–102. [Google Scholar] [CrossRef]
- Wada, M.; Kira, M.; Kido, H.; Ikeda, R.; Kuroda, N.; Nishigaki, T.; Nakashima, K. Semi-micro flow injection analysis method for evaluation of quenching effect of health foods or food additive antioxidants on peroxynitrite. Luminescence 2010, 26, 191–195. [Google Scholar] [CrossRef]
- Ruzicka, J. Flow injection analysis? A survey of its potential as solution handling and data gathering technique in chemical research and industry. Anal. Bioanal. Chem. 1988, 329, 653–655. [Google Scholar] [CrossRef]
- Vasilescu, A.; Gheorghiu, M.; Peteu, S. Nanomaterial-based electrochemical sensors and optical probes for detection and imaging of peroxynitrite: A review. Microchim. Acta 2017, 184, 649–675. [Google Scholar] [CrossRef]
- Vasilescu, A.; Dinca, V.; Filipescu, M.; Rusen, L.; Hosu, I.S.; Boukherroub, R.; Szunerits, S.; Dinescu, M.; Peteu, S. CHAPTER 9. Recent Approaches to Enhance the Selectivity of Peroxynitrite Detection. In Detection Science; Royal Society of Chemistry (RSC): London, UK, 2015; pp. 166–185. [Google Scholar]
- Amatore, C.; Arbault, S.; Bruce, D.; de Oliveira, P.; Erard, M.; Vuillaume, M. Characterization of the electrochemical oxidation of peroxynitrite: Relevance to oxidative stress bursts measured at the single cell level. Chem. Eur. J. 2001, 7, 4171–4179. [Google Scholar] [CrossRef]
- Koh, W.C.A.; Son, J.I.; Choe, E.S.; Shim, Y.-B. Electrochemical Detection of Peroxynitrite Using a Biosensor Based on a Conducting Polymer−Manganese Ion Complex. Anal. Chem. 2010, 82, 10075–10082. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F.; Corbalan, J.J.; Szczesny, D.; Matysiak, K.; Malinski, T. The favorable kinetics and balance of nebivolol-stimulated nitric oxide and peroxynitrite release in human endothelial cells. BMC Pharmacol. Toxicol. 2013, 14, 48. [Google Scholar] [CrossRef]
- Cortés, J.S.; Granados, S.G.; Ordaz, A.A.; Jiménez, J.A.L.; Griveau, S.; Bedioui, F. Electropolymerized Manganese Tetraaminophthalocyanine Thin Films onto Platinum Ultramicroelectrode for the Electrochemical Detection of Peroxynitrite in Solution. Electroanalysis 2007, 19, 61–64. [Google Scholar] [CrossRef]
- Hosu, I.S.; Constantinescu-Aruxandei, D.; Jecu, M.-L.; Oancea, F.; Doni, M. Peroxynitrite Sensor Based on a Screen Printed Carbon Electrode Modified with a Poly(2,6-dihydroxynaphthalene) Film. Sensors 2016, 16, 1975. [Google Scholar] [CrossRef]
- Hosu, I.S.; Wang, Q.; Vasilescu, A.; Peteu, S.F.; Raditoiu, V.; Railian, S.; Zaitsev, V.; Turcheniuk, K.; Wang, Q.; Li, M.; et al. Cobalt phthalocyanine tetracarboxylic acid modified reduced graphene oxide: A sensitive matrix for the electrocatalytic detection of peroxynitrite and hydrogen peroxide. RSC Adv. 2015, 5, 1474–1484. [Google Scholar] [CrossRef]
- Boni, A.C.; Wong, A.; Dutra, R.A.F.; Sotomayor, M.D.P.T. Cobalt phthalocyanine as a biomimetic catalyst in the amperometric quantification of dipyrone using FIA. Talanta 2011, 85, 2067–2073. [Google Scholar] [CrossRef]
- Robinson, K.M.; Beckman, J.S. Synthesis of Peroxynitrite from Nitrite and Hydrogen Peroxide. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 2005; Volume 396, pp. 207–214. [Google Scholar]
- Singh, R.J.; Hogg, N.; Joseph, J.; Konorev, E.; Kalyanaraman, B. The Peroxynitrite Generator, SIN-1, Becomes a Nitric Oxide Donor in the Presence of Electron Acceptors. Arch. Biochem. Biophys. 1999, 361, 331–339. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Al-Mehdi, A.B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995, 364, 279–282. [Google Scholar] [CrossRef]
- Shih, Y. Flow injection analysis of zinc pyrithione in hair care products on a cobalt phthalocyanine modified screen-printed carbon electrode. Talanta 2004, 62, 912–917. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Sreedhar, B.; Sain, B.; Ray, S.S.; Jain, S.L. Cobalt Phthalocyanine Immobilized on Graphene Oxide: An Efficient Visible-Active Catalyst for the Photoreduction of Carbon Dioxide. Chem. A Eur. J. 2014, 20, 6154–6161. [Google Scholar] [CrossRef]
- Tackley, D.R.; Dent, G.; Smith, W.E. IR and Raman assignments for zinc phthalocyanine from DFT calculations. Phys. Chem. Chem. Phys. 2000, 2, 3949–3955. [Google Scholar] [CrossRef]
- Foster, C.W.; Pillay, J.; Metters, J.P.; Banks, C.E. Cobalt Phthalocyanine Modified Electrodes Utilised in Electroanalysis: Nano-Structured Modified Electrodes vs. Bulk Modified Screen-Printed Electrodes. Sensors 2014, 14, 21905–21922. [Google Scholar] [CrossRef]
- Kozub, B.R.; Compton, R.G. Voltammetric studies of the redox mediator, cobalt phthalocyanine, with regard to its claimed electrocatalytic properties. Sensors Actuators B Chem. 2010, 147, 350–358. [Google Scholar] [CrossRef]
- Herold, S.; Exner, M.; Boccini, F. The Mechanism of the Peroxynitrite-Mediated Oxidation of Myoglobin in the Absence and Presence of Carbon Dioxide. Chem. Res. Toxicol. 2003, 16, 390–402. [Google Scholar] [CrossRef]
- Rehmann, F.-J. Mechanistic Studies of the Nitrogen Monoxide-and Nitrite-Mediated Reduction of Ferryl Myoglobin and Ferryl Hemoglobin. Doctoral Dissertation, Naturwissenschaften ETH Zürich, Zürich, Switzerland, 2002. [Google Scholar]
- Dashteh, M.; Safaiee, M.; Baghery, S.; Zolfigol, M.A. Application of cobalt phthalocyanine as a nanostructured catalyst in the synthesis of biological henna-based compounds. Appl. Organomet. Chem. 2019, 33, e4690. [Google Scholar] [CrossRef]
- Møinichen, C. Microfluidic flow cells for studies of electrochemical reactions. Master’s Thesis, Institutt for materialteknologi, Urgench, Uzbekistan, 2012. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosu, I.S.; Constantinescu-Aruxandei, D.; Oancea, F.; Doni, M. Studying the Reaction of Peroxynitrite with Myoglobin for Meat Extract Samples Using Cobalt Phthalocyanine-Modified Screen-Printed Carbon Electrodes and a Flow Injection Analysis System. Proceedings 2020, 60, 46. https://doi.org/10.3390/IECB2020-07310
Hosu IS, Constantinescu-Aruxandei D, Oancea F, Doni M. Studying the Reaction of Peroxynitrite with Myoglobin for Meat Extract Samples Using Cobalt Phthalocyanine-Modified Screen-Printed Carbon Electrodes and a Flow Injection Analysis System. Proceedings. 2020; 60(1):46. https://doi.org/10.3390/IECB2020-07310
Chicago/Turabian StyleHosu, Ioana Silvia, Diana Constantinescu-Aruxandei, Florin Oancea, and Mihaela Doni. 2020. "Studying the Reaction of Peroxynitrite with Myoglobin for Meat Extract Samples Using Cobalt Phthalocyanine-Modified Screen-Printed Carbon Electrodes and a Flow Injection Analysis System" Proceedings 60, no. 1: 46. https://doi.org/10.3390/IECB2020-07310
APA StyleHosu, I. S., Constantinescu-Aruxandei, D., Oancea, F., & Doni, M. (2020). Studying the Reaction of Peroxynitrite with Myoglobin for Meat Extract Samples Using Cobalt Phthalocyanine-Modified Screen-Printed Carbon Electrodes and a Flow Injection Analysis System. Proceedings, 60(1), 46. https://doi.org/10.3390/IECB2020-07310






