1. Introduction
There is increasing evidence that oxidative stress, defined as an imbalance between levels of various oxidant molecules and antioxidants, leads to many biochemical changes and, consequently, serious disorders in the human organism. Oxidative stress can result in damage of biomolecules, such as lipids, proteins and DNA, leading to cytotoxic and genotoxic effects [
1,
2].
Today, it is evident that mutagenicity and other adverse effects of reactive oxygen species (ROS) are implicated in aging, atherosclerosis, cancer, diabetes, and several neurodegenerative diseases [
3,
4,
5,
6].
The reaction of most toxic hydroxyl HO
• radicals is mainly an addition to the double bonds of pyrimidine bases and abstraction of hydrogen from the sugar moiety resulting in DNA chain scission. DNA lesions resulting from attack by reactive oxygen species (ROS) can be a major cause of mutagenesis and carcinogenesis [
7].
The study of new sources of natural antioxidants has received increasing attention in the last decades. Many essential oils and their constituents have recently been qualified as natural antioxidants and proposed as potential substitutes for synthetic ones [
8,
9,
10,
11]. Due to their radical scavenging properties, antioxidants are believed to be directly antimutagenic and anticarcinogenic [
12].
The genus
Schinus L. (Anacardiaceae) is represented in Argentina by 22 species.
S. areira L. (syn.
Schinus molle L. var.
areira (L.) DC.): is a native species frequently planted as an ornamental and for shade. It originates from the northwest of Argentina and it is commonly known as “aguaribay”, “árbol de la pimienta”, “pepper tree”, “terebinto” or “pimientero”. It is native in Bolivia, Southern Brazil, Paraguay and Northern Argentina, but is now becoming established in most temperate regions of the world, having been distributed very early by Spanish colonists [
13,
14,
15]. Leaves, fruits, stems and bark, dried or fresh, are used to prepare infusions, ointments, cataplasms, beverages, collyrium, etc, which are used as purgative, diuretic, vulnerary, topic disinfectant and for the treatment of rheumatism, stomach upset, menstrual disorders, gonorrhea, bronchitis and conjunctivitis [
16,
17].
Globulol is a sweet, fruity, and green tasting compound and can be found in a number of food items such as peppermint, orange mint, spearmint, and sweet bay, which makes globulol a potential biomarker for the consumption of these food products [
18]. Globulol has been reported as an active sesquiterpenoid with moderate antioxidant ability [
19]. This compound has antifungal activity against a variety of fungal species, as well as bacterial ones [
20]. Furthermore, globulol has spasmolytic activity [
21].
The antioxidant activity of the EOs and one of its components, globulol, was evaluated by two complementary test systems: reduction of the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [
22], and crocin bleaching inhibition method [
23].
One of the important short time test systems used for the determination of chemically induced mutations at cell level, is the Ames/Salmonella microsome test system. This system constitutes a great potential in terms of the determination of carcinogenic substances and prevention of their risks following the presentation of a strong relationship between the mutagenic effects of chemical substances and the development of cancer. Ames test system is also used for the determination of antimutagens and anticarcinogens, which eliminate the mutagenic or carcinogenic effects of chemicals and prevent the interaction of these chemicals with DNA [
24].
The antimutagenic and antipromutagenic activities were evaluated in vitro and ex vivo, using the Ames assay with five strains of Salmonella typhimurium (TA98, TA100, TA1535, TA1537 and TA102) with and without exogenous metabolic activation (rat liver fraction S9), against different mutagens (sodium azide (AZ), 2,4,7-trinitro-9-fluorenone (TNF), 9-aminoacridine (9AA), 2-aminoanthracene (2AA), t-butyl hydroperoxide (t-BuOOH) and hydrogen peroxide (H2O2). It was observed that the samples were able to reduce or inhibit DNA damage or mutations induced by cancer cells and disease caused by genotoxic agents.
The aim of this work was to further evaluate the properties of essential oils from leaves of Schinus areira and one of its components, globulol, such as antimutagenic, antipromutagenic and antioxidant activities.
2. Material and Methods
2.1. General
Gas chromatography-mass spectrometry (GC-MS) analyses were performed with a Hewlett-Packard 6890 chromatograph connected to a Hewlett-Packard 5972A mass spectrometer equipped with a capillary column (HP-5, 25 m × 0.25 mm, 0.25 µm film thickness). The carrier gas was helium with flow 1 mL/min. The GC oven temperature was held at 50 °C for 2 min, programmed at 5 °C/min to 200 °C, then held at this temperature for 15 min. Mass spectra were recorded at 70 eV. Mass range was from m/z 35–350 amu. The temperature of the injection block was 250 °C.
GC analyses were performed on a Shimadzu G14B chromatograph with a flame ionization detector on a DB-5 column (30 m × 0.25 mm, 0.25 µm film thickness) with the same analytical conditions used for the GC-MS analyses. UV spectra were recorded on a GBC Spectral UV-VIS spectrophotometer.
Nuclear Magnetic Resonance (NMR) spectroscopy were recorded with a Bruker ARX300 spectrometer operated at 300 and 150 MHz for 1H and 13C, respectively, in CDCl3. Chemical shifts are given in ppm (δ) with tetramethylsilane (TMS) as an internal standard.
2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2-azobis (2-aminopropane) dihydrochloride (AAPH), and crocin were purchased from Sigma-Aldrich. Silica gel 60 F254 plates (0.2 mm thickness) were purchased from Merck.
2,4,7-trinitro-9-fluorenone (TNF), sodium azide (AZ), t-butylhydroperoxide (t-BuOOH), hydrogen peroxide (H2O2), 9-aminoacridine (9AA), 2-aminoanthracene (2AA) were purchased from Aldrich.
Phosphate buffer (Na2HPO4 and NaH2PO4·H2O), reduced nicotinamide adenine dinucleotide phosphate (NADPH), glucose-6-phosphate, dimethyl sulfoxide (DMSO), biotin, histidine, ampicillin, solution of salts (MgCl2 and KCl) were purchased from Sigma-Aldrich. The S9 fraction (S9 microsomal fraction of homogenized rat liver aroclor 1254, MOLTOX®, Molecular Toxicology Inc., Boone, NC, USA).
2.2. Plant Material
Aerial parts of S. areira (leaves) were collected at Bahía Blanca city, Buenos Aires province, Argentina, in March 2006. The taxonomy of this material was determined by Dr. M.G. Murray. Voucher specimens are kept in the “Herbario del Departamento de Biología, Bioquímica y Farmacia-Universidad Nacional del Sur (BBB)” under the numbers MGM121.
2.3. Essential Oil and Globulol Obtention
Essential oil was obtained using hydrodistillation as previously reported (Murray et al. 2005) [
25]. The chemical composition of oil was determined using GC and GC-MS. The compounds were identified by comparison of their retention indices (Kovats indices) with those of known compounds and also by comparison of their mass spectra with those stored in the MS databases (NBS75K.L MS DATA). Relative percentage amounts were obtained directly from GC peak areas. Results are summarized in
Table 1.
The EOs was fractionated using reverse-phase preparative chromatography (RP-HPLC) and it was performed on an RP-HPLC Konik 500A. The EOs was dissolved in methanol for the chromatography analysis. For this, 300 μL of an oil solution in MeOH (1:3 (v/v)) was injected. An isocratic mobile phase (methanol: water, 90:10) condition was maintained throughout the process, in 30 min. The samples were eluted from the column (C18 Phenomenex Gemini 5 µm C18 110 Å, 250 × 10 mm) at room temperature with a flow rate of 1 mL/min, which was detected at 254 nm and fractions EO1 - EO5 were obtained. EO2 (4.8 mg) was eluted with a retention time of 24.5 min to furnish the active compound identified as globulol. The compound was analyzed using GC/MS and it was identified by comparison of their retention indices (Kovats indices) and also by comparison of their MS with those stored in the MS database (NBS75K.L MS DATA). Relative percentage amounts were obtained directly from GC peak areas. The identity of this compound was confirmed by its 1H and 13C NMR spectra, recorded in CDCl3 on a Bruker ARX 300 spectrometer at 300 MHz and 75 MHz, respectively, according to the literature.
2.4. Antimutagenic Activity
Mutagenicity, antimutagenicity and antipromutagenicity assays: Plate incorporation tests were performed as previously described (Maron and Ames, 1983) using S. typhimurium tester strains (TA98, TA100, TA1535, TA1537 and TA102) with and without an exogenous metabolic system (S9). Bacterial strains of S. typhimurium were kindly provided by Microbiology Laboratory, USC, Spain.
Different concentrations were prepared ranging from 0.0005 to 0.5 μg/plate of EOs and globulol, which were used for mutagenicity, antimutagenicity and antipromutagenicity assays against known positive mutagens, with or without metabolic activation (S9).
Briefly, 100 μL of bacterial stock were incubated in 20 mL of nutrient broth for 16 h at 37 °C on a rotative shaker. One hundred microliters of this overnight culture was mixed with 2.0 mL of top agar (containing histidine-biotin) together with 0.1 mL test solution and 0.5 mL phosphate buffer.
For mutagenicity screening, the test solution contained 50 μL test sample and 50 μL solvent control. For antimutagenicity screening, the test solution contained 50 μL test sample and 50 μL positive control. The top agar mixture was poured over the surface of a minimal agar plate and incubated for 48 h at 37 °C. After incubation, the numbers of revertant colonies (mutants) in each plate were counted.
Antimutagenicity was expressed as percentage inhibition of mutagenicity calculated using the formula below.
where T is the number of revertants per plate in the presence of mutagen and the test solution and M is the number of revertants per plate in the positive control. All cultures were prepared in triplicate.
A similar experiment was also carried out using liver homogenate (S9) fractions. The reagent 2-aminoanthracene (2AA) was used as a positive control in analysis systems with metabolic activation of all of the S. typhimurium strains. For analysis systems without metabolic activation and as a positive control, 2,4,7-trinitro-9-fluorenone and 9-aminoacridine were employed in the assays with strains TA98 and TA1537, respectively. Sodium azide was employed in the assays with strains TA1535 and TA100; t-butylhydroperoxide and hydrogen peroxide were used in the assays with the strain TA102.
Phosphate buffer was used as a negative control of analysis systems with and without metabolic activation with all S. typhimurium strains.
Absence of toxicity was confirmed when a background layer of bacterial growth was observed, which should normally be present. For all the samples tested in the current experiments, the density of background bacterial lawn was compared to that of the negative control (after 48 h) and found to have no visible differences, indicating a lack of toxicity to the bacteria at the concentration tested.
Preparation of S9 fraction: The procedure used for the preparation of rat liver homogenate S9 was followed as reported in literature (Ames et al., 1973). All the steps were performed at 0 °C to 4 °C with cold and sterile solutions and glassware. The S9 fractions were distributed in 2 mL aliquots in small sterile plastic tubes, quickly frozen and stored at −80 °C. The S9 mix was prepared following the reported method (Maron and Ames, 1983).
2.5. Antioxidant Activity
Crocin bleaching inhibition assay: The antioxidant capacity was evaluated according to the protocol of Tubaro et al. [
23]. Briefly, the reaction mixture contained 10 μM crocin and increasing amounts of samples (from 10 to 100 μM). The reaction was started by the addition of 10 mM AAPH to the reaction mixture preequilibrated at 40 °C. The bleaching rate of crocin (
Vo), that is, the rate of its reaction with peroxyl radicals, was calculated by measuring the decrease of its absorption at 443 nm in the first 10 min of reaction, using a spectrophotometer equipped with a thermostable cell block, against a blank. In the presence of various concentrations of samples, the corresponding bleaching rates were termed
Va. Each kinetic analysis was compared with a kinetic crocin bleaching.
The slopes, calculated by linear regression analysis of the plot [antioxidant]/[crocin] versus Va/Vo, indicate the relative capacities of the different molecules to interact with ROO•. The antioxidant capacity of the essential oil and globulol, relative to the activity of Trolox, was calculated by dividing the slope of each compound by the slope of Trolox. Three replicates were made for each sample.
DPPH free radical-scavenging assay: The antioxidant activity of the essential oil and globulol was evaluated through its ability as a free radical scavenger. The preliminary test was performed with a rapid thin-layer chromatography (TLC) screening method using the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH). Analytical TLC on silica gel plates were developed with appropriate conditions after application of 5 µL of oil solution (5 mg/mL) and dried and sprayed with DPPH solution (0.2%, MeOH). Five minutes later, active compounds appeared as yellow spots against a purple background. Trolox (1 mg/mL) was used as positive control.
The DPPH free radical scavenging spectrophotometric method described by Bors et al. [
22] was used to determine the quantitative antioxidant activity. Reactions were carried out in 96-well microtiter plates, and each of the samples was tested at different concentrations. Blank solutions were prepared with methanol while the negative control was the DPPH solution (0.002%, MeOH). Test sample solution (150 μL) contained EOs serially diluted in methanol. Methanol served as a blank for the microplate reader, and the decrease in absorbance was measured at 517 nm. Percentage antioxidant activity (AA%) values were calculated from the absorbance values using the formula:
(Abs sample is the absorbance of the sample, Abs blank is the absorbance of the blank and Abs control is the absorbance of the control).
Trolox was used as a positive control. The IC50 value, defined as the concentration of the sample leading to 50% reduction of the initial DPPH concentration, was calculated from the separate linear regression of plots of the mean percentage of the antioxidant activity against concentration of the test EOs obtained from the three replicate assays. The results are expressed as IC50 values obtained from the regression plots.
2.6. Statistical Analysis
Statistical analysis was performed using Tukey‒Kramer correction of a one-way ANOVA test using Graph-Pad Prism 5.0 software (GraphPad Software, San Diego, CA, USA).
3. Results and Discussion
3.1. Essential Oil Composition
The essential oil, obtained from fresh plant material, was analyzed using gas chromatography and mass spectrometry, leading to the identification of 32 components, which are listed in
Table 1. The identification of each volatile compound was achieved by comparing its retention index with those cited in the literature, its mass spectrum with those of the database, and its retention time with authentic samples, when available.
The major components were α-felandrene (16.18%), β-felandrene (11.47%) and globulol (16.61%). According to their functional groups, the most abundant types were hydrocarbons (67.96%), alcohols (30.61%) and esters (1.24%). The essential oil possesses a high percentage of hydrocarbons monoterpenes (56.05%), followed by oxygenated sesquiterpenes (30.61%), hydrocarbons sesquiterpenes (11.92%) and oxygenated monoterpenes (1.42%).
The EOs were fractionated by reverse-phase preparative chromatography (RP-HPLC). This allowed the isolation of the active compound globulol (tR 24.5 min).
The major constituent (16.61%) of the oil was identified as globulol by comparison of its mass spectra EI-MS
m/
z (%): 222 (M
+) with that stored in the MS database (NBS75K.L MS DATA) [
26]. The identity of this compound was confirmed by its
1H and
13C NMR spectra, recorded in CDCl
3 on a Bruker ARX 300 spectrometer at 300 MHz and 75 MHz, respectively, according to the literature [
27].
3.2. Antimutagenic Activity
The tester strains were selected for their sensitivity and specificity to being reverted to prototrophy by different types of mutational events. The strains TA98 and TA1537 can be used to detect mutagens causing frameshift mutations, while TA100 and TA1535 are used to detect mutagens causing base pair substitutions in bacterial DNA. The samples were tested in the TA102 strain, which is susceptible to oxidative stress-induced mutation [
28].
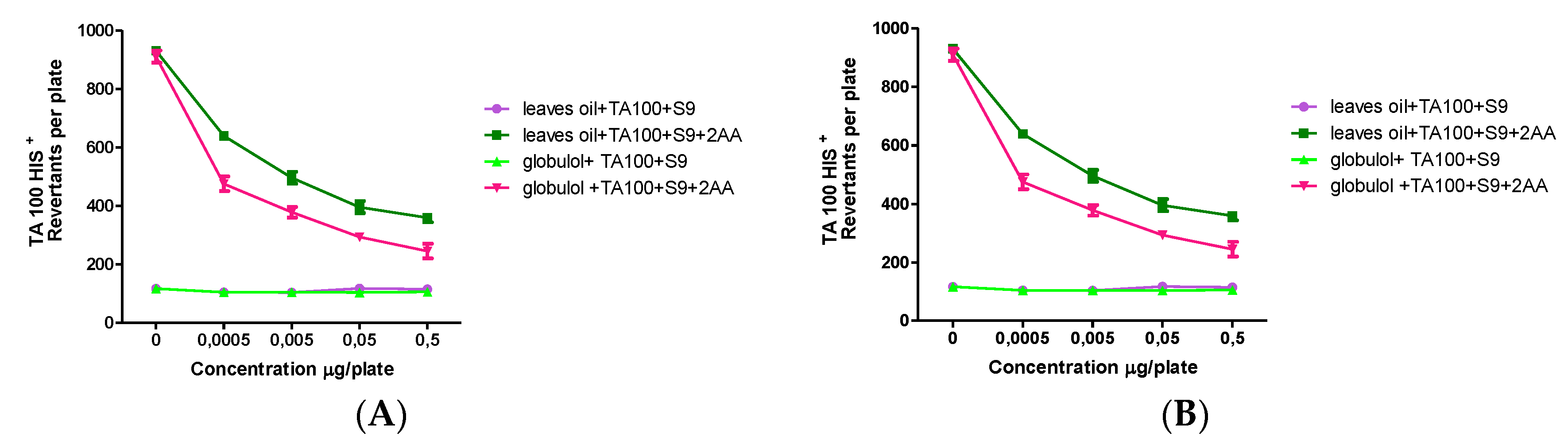
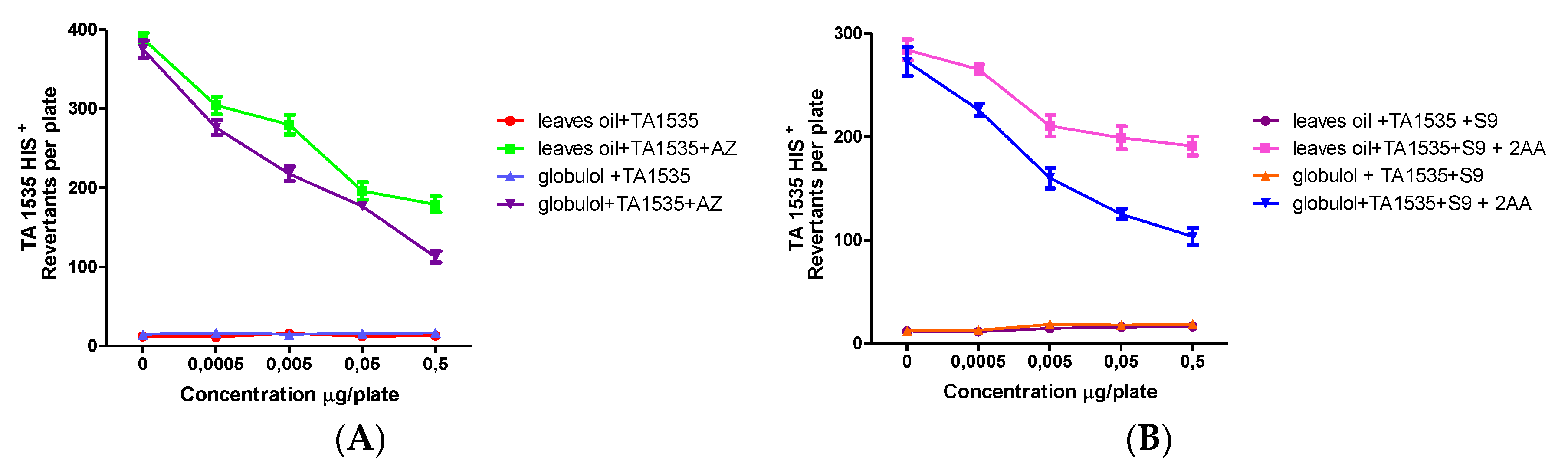
Table 2 gives an overview on the obtained data in the plate incorporation assay.
The result determined that the major compound and EOs at the applied doses do not exhibit significant increase (
p < 0.05) in the number of revertant colonies of the
S. typhimirium strains TA98, TA100, TA102, TA1537 and TA1535, in the absence and presence of an exogenous metabolic activation system, at concentrations between 0.5 and 0.0005 μg/plate. The doses of EOs and globulol used in this work were not toxic for the strains, when evaluated alone. Procedures included the use of positive and negative controls, standard procedures for performing the assay, with or without S9 mix, checking the tester strains for genetic and physiological stability and evaluating the results statistically [
29].
The EOs and globulol also showed antimutagenic and antipromutagenic activities against different mutagens (sodium azide (AZ), 9 aminoacridine (9AA), 2,4,7-trinitro-9-fluorenone (TNF), 2-aminoanthracene (2AA), t-butyl hydroperoxide (t-BuOOH) and hydrogen peroxide (H2O2).
The inhibition of the mutagenic effect from EOs and globulol ranged from 30.2–78.3% to 39.5–92.9% respectively, in a concentration-dependent manner, while the antipromutagenic activity of EOs and globulol resulted in a range from 41.2–85.4% to 46.9–98.3%, respectively (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
The EOs exhibited strong antimutagenic activity, statistically significant, at all applied dosage levels. This could be associated with the presence of globulol, the main compound in the oil which is reported for the first time as a potent antimutagenic.
The antipromutagenic activity was evaluated ex vivo, using the Ames assay with five strains of S. typhimurium with exogenous metabolic activation (rat liver fraction S9), against 2AA mutagen. It was observed that the samples were able to reduce mutations induced, which indicated a deactivation by the liver enzymes.
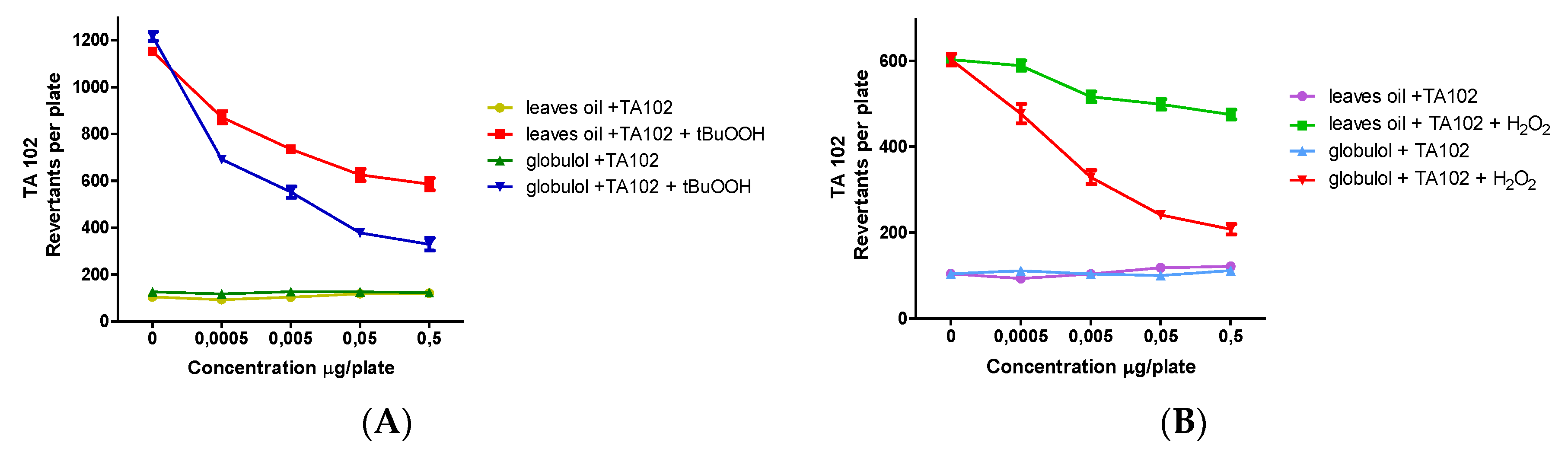
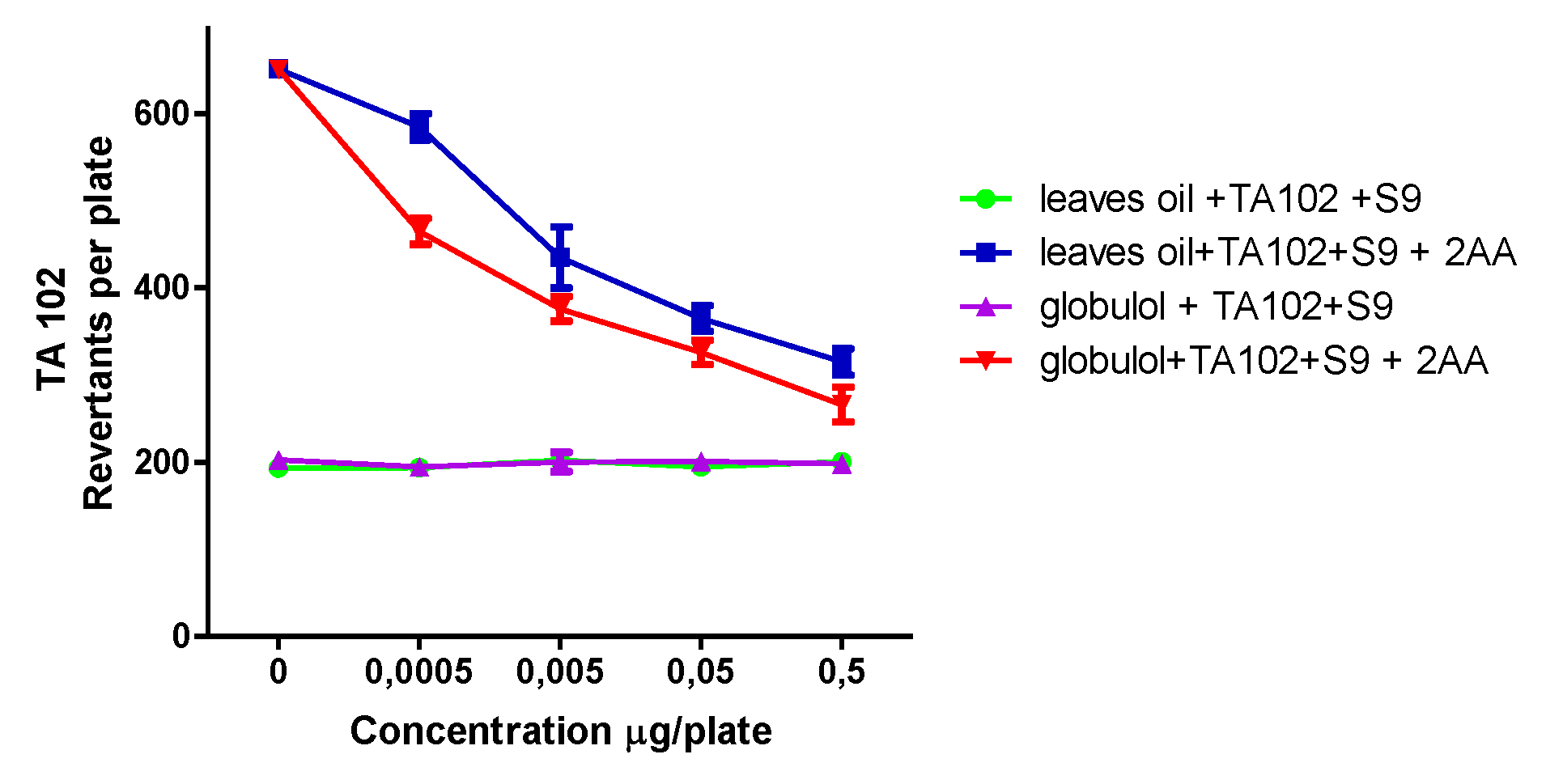
Possible antioxidant effects of EOs and globulol in the absence of metabolic activation were tested after stressing with H2O2 or t-BuOOH. In order to prove the oxidative DNA damage, the S. typhimurium strain TA102 was used, which is highly sensitive toward ROS.
Hydrogen peroxide possesses high membrane permeability, and it is not only a reducing agent but also an oxidizing reagent and acts mainly via the Fenton reaction, thereby generating hydroxyl radicals. Under the influence of H2O2, EOs and globulol showed a significant antimutagenic effect with IC50 values of 38.52 ± 2.41 µg/mL and 3.42 ± 0.20 µm, respectively.
As different mechanisms of oxidative DNA damage exist,
t-BuOOH was used as a second oxidant. It is known as an initiator of lipid peroxidation and leads to the formation of alkoxyl and alkyl radicals [
30]. Antioxidant testing in the TA102 strain revealed that EOs and globulol could effectively inhibit the genotoxic effect of
t-BuOOH-induced oxidative stress, with IC
50 values of 12.05 ± 2.10 µg/mL and 2.18 ± 0.23 µm, respectively. The apparent antioxidant and antimutagenic behavior of both samples further suggests their presence in biological systems is of possible physiological importance.
All of these Ames test results showed that both EOs and globulol were not genotoxic regarding frameshift mutations (TA98 and TA1537) and base pair substitutions (TA100, TA102, TA1535). Thus, the samples were able to reduce or inhibit DNA damage or mutations induced by cancer cells and disease caused by genotoxic agents.
The significant antimutagenic activity of globulol against all mutagens suggests that this compound may directly protect DNA damage from mutagen, and activity against t-BuOOH and H2O2 confirmed their antioxidant potential.
3.3. Antioxidant Activity Assay
The “crocin bleaching assay” (CBA) has been applied for the evaluation of the antioxidant capacity of individual compounds, plant extracts, and plasma [
31]. This method is interesting because it can be used in both lipophilic and hydrophilic environments [
32] and is based on the bleaching of crocin as a result of its oxidation by a source of radicals, AAPH (2,2-azo-bis (2-aminopropane) dihydrochloride). In the present work, peroxyl radical scavenging was evaluated according to the protocol of Tubaro et al. [
23]. These studies demonstrated that the essential oil of
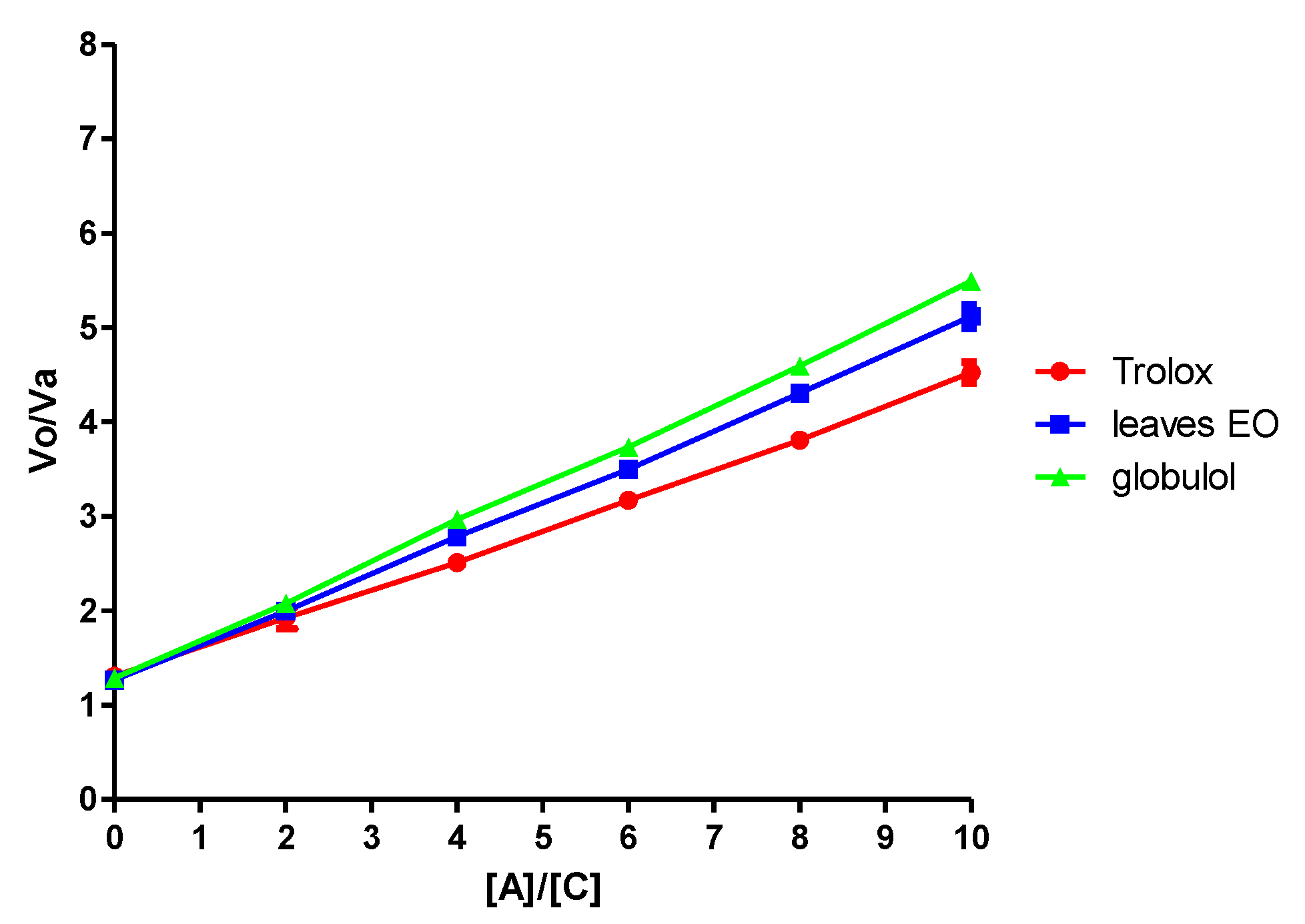
S. areira and its active component, globulol, possesses potent peroxyl radical scavenger activity with a Trolox Equivalent Value (TEV Krel) of 1.16 ± 0.11 and 1.24 ± 0.22, respectively. The results obtained for each sample are summarized in
Figure 7 and
Table 3.
3.4. DPPH Free Radical Scavenging
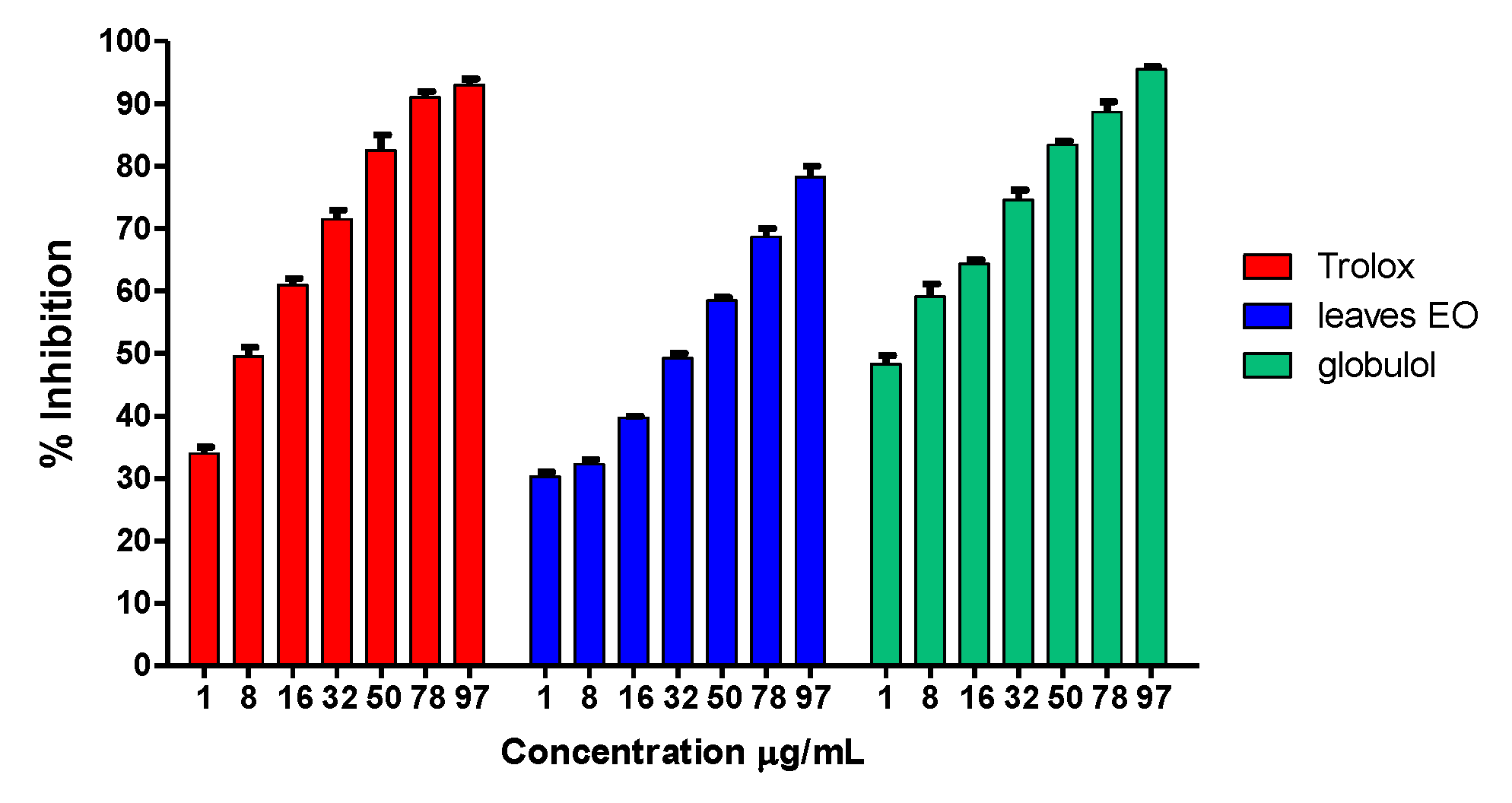
The antioxidant activity of the essential oil was also evaluated through its ability as a free radical scavenger against DPPH. The preliminary test was performed using a rapid TLC screening method in order to detect the antioxidant activity. Then, a spectrophotometric assay was carried out at different concentrations of the essential oil and globulol. The percentage of DPPH reduction was calculated taking into account the absorbance of the blank solutions and the negative control. Trolox was used as reference compound under the same experimental conditions. Essential oil from S. areira and globulol elicited marked radical scavenger activity (IC50 = 38.75 ± 2.5 μg/mL vs. 5.60 ± 0.91 μg/mL), with an antioxidant capacity comparable to that of Trolox (IC50 = 7.67 ± 1.02 μg/mL).
The results obtained for each sample are summarized in
Figure 8 and
Table 4.
The antioxidant activity observed for the
S. areira oil could be explained by the high percentage active sesquiterpene globulol (16.61%) present in this oil and also by the presence of sabinene (4.27%). Globulol has been reported as an active sesquiterpenoid with moderate antioxidant ability, while the monoterpene sabinene has shown notorious antioxidant ability [
19].
The results obtained in both the DPPH and crocin assays demonstrate that the essential oil of this plant has marked antioxidant activity that could be explained by the presence of components like camphene (6.27%) [
33] and α-pinene (5.42%) [
34]. The presence of other antioxidant compounds, even though at low concentrations, like β-myrcene (3.45%), β-eudesmol (0.53%) and the potent antioxidant bornyl acetate (1.41%), may also contribute to the radical scavenging activity of this oil [
35].
4. Conclusions
Globulol and the essential oil obtained from S. areira can act by several antimutagenic mechanisms. They are able to reduce alkylated DNA damage through a reduction in the expression of base-substitution mutations. Besides, these mechanisms are antimutagenic by reducing the activation of mutagens like 2AA, and they act against H2O2 and t-BuOOH as possible ROS-scavenging mixtures, as proven by DPPH and crocin bleaching assays.
The results of this work prove that the active compound, globulol, and S. areira essential oil could be useful as potential sources of natural, safe and efficient antioxidants, as antimutagenic and/or chemo preventive agents, for both the pharmaceutical and food industries. The active compound, globulol, which was isolated here, could became a leading compound for the development of new drugs to improve the treatment of several diseases, since antioxidant therapy will help to avoid the neuronal degeneration and cellular aging that free radicals provoke.