Synthesis One Pot of Alkyne-2-Chloroquinoline via a Passerini Reaction †
Abstract
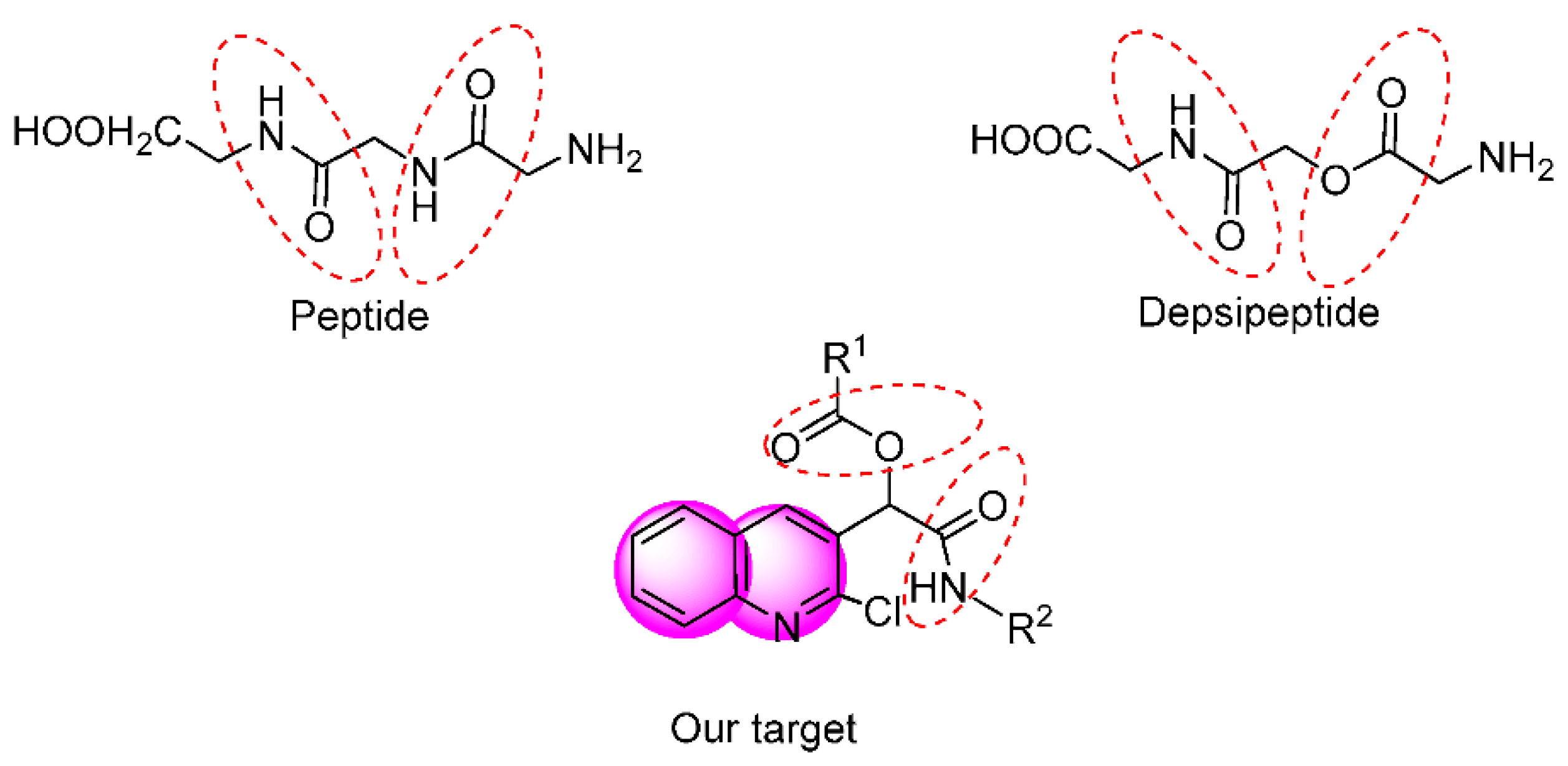
:1. Introduction
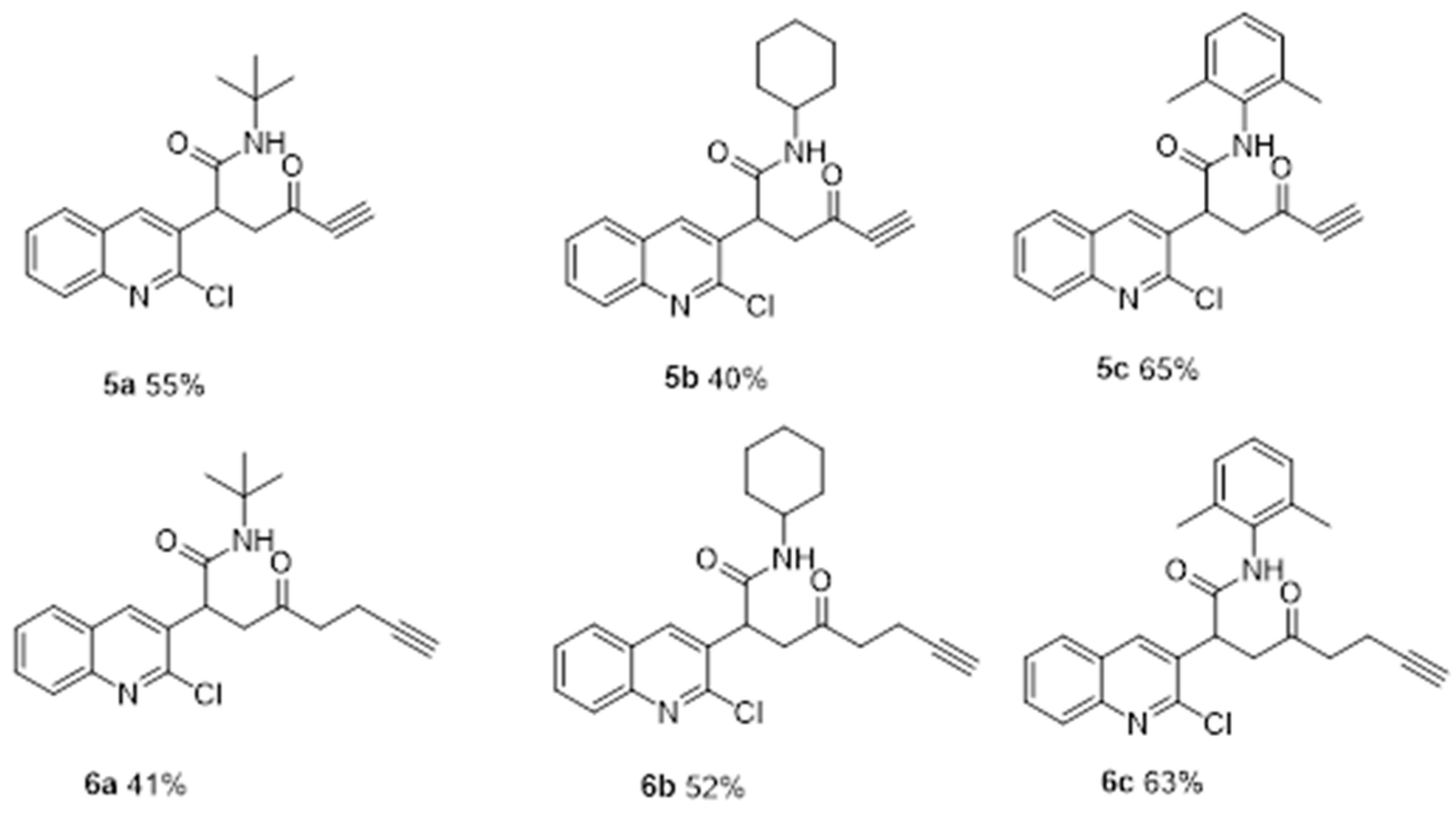
2. Results and Discussion
3. Experimental Section
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Bienayme, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978-3-527-30806-4. [Google Scholar]
- Orru, R.V.A.; de Greef, M. Recent Advances in solution-Phase Multicomponent Methodology for the synthesis of Heterocyclic Compounds. Synthesis 2003, 10, 1471–1499. [Google Scholar] [CrossRef]
- Zhu, J. Recent Developments in the isonitrile-Based Multicomponent Synthesis of Heterocycles. Eur. J. Org. Chem. 2003, 1133–1144. [Google Scholar] [CrossRef]
- Kazemizade, A.R.; Ramazani, A. Synthetic Applications of Passerini Reaction. Curr. Org. Chem. 2012, 16, 418–450. [Google Scholar] [CrossRef]
- Ugi, I.; Meyer, R. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Kitagaki, J.; Shi, G.; Miyauchi, S.; Murakami, S.; Yang, Y. Cyclic depsipeptides as potencial cancer therapeutics. Anti-Cancer Drugs 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Pirrung, M. Multicomponent Reactions are accelerated in water. J. Am. Chem. Soc. 2004, 126, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.K.Z.; Takada, S.C.S.; Suarez, P.A.Z.; Alves, M.B. Revisiting the Passerini reaction under eco-friendly reaction conditions. Synlett 2006, 10, 1539–1542. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Zhang, X.; Qu, G.; Wang, J. A novel and green version of the Passerini reaction in an ionic liquid ([bmim] [BF4]). Can. J. Chem. 2006, 84, 794–799. [Google Scholar] [CrossRef]
- Shaabani, A.; Afshari, R.; Hooshmand, S.E. Passerini three-component cascade reactions in deep eutectic solvent: An environmentally benign and rapid system for the synthesis of α-acyloxyamides. Res. Chem. Intermed. 2015, 42, 5607–5616. [Google Scholar] [CrossRef]
- Koszelewski, D.; Szymanski, W.; Krysiak, J.; Ostaszewski, R. Solvent-free Passerini reactions. Synth. Commun. 2008, 38, 1120–1127. [Google Scholar] [CrossRef]
- Polindara-García, L.A.; Juaristi, E. Synthesis of Ugi 4-CR and Passerini 3-CR adducts under mechanochemical activation. Eur. J. Org. Chem. 2016, 1095–1102. [Google Scholar] [CrossRef]
- Barreto, A.F.S.; Vercillo, O.E.; Andrade, C.K.Z. Microwave-Assisted Passerini reactions under solvent-free conditions. J. Braz. Chem. Soc. 2011, 22, 462–467. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, C.; Bian, Q.; Cui, C.; Du, X.J.; Li, Z.M.; Zhao, W.G. Novel ultrasound-promoted parallel synthesis of trifluoroatrolactamide library via a One-pot Passerini/hydrolysis reaction sequence and their fungicidal activities. ACS Comb. Sci. 2014, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, F.; Crosignani, S.; Galatini, A.; Riva, R.; Basso, A. Novel Application of α-Azido Aldehydes in Multicomponent Reactions: Synthesis of Triazolo-Fused Dihydrooxazinones via a Passerini Reaction–Dipolar Cycloaddition Strategy. ACS Comb. Sci. 2011, 13, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Aragoncillo, C.; Callejo, R.; Ruiz, M.P.; Torres, M.R. Regio- and Diastereoselective Synthesis of β-Lactam-Triazole Hybrids via Passerini/CuAAC Sequence. J. Org. Chem. 2012, 77, 6917–6928. [Google Scholar] [CrossRef] [PubMed]
- René, O.; Fagnau, K. Room-temperature direct arylation of polyfluorinated arenes under biphasic conditions. Org. Lett. 2010, 12, 2116–2119. [Google Scholar] [CrossRef] [PubMed]

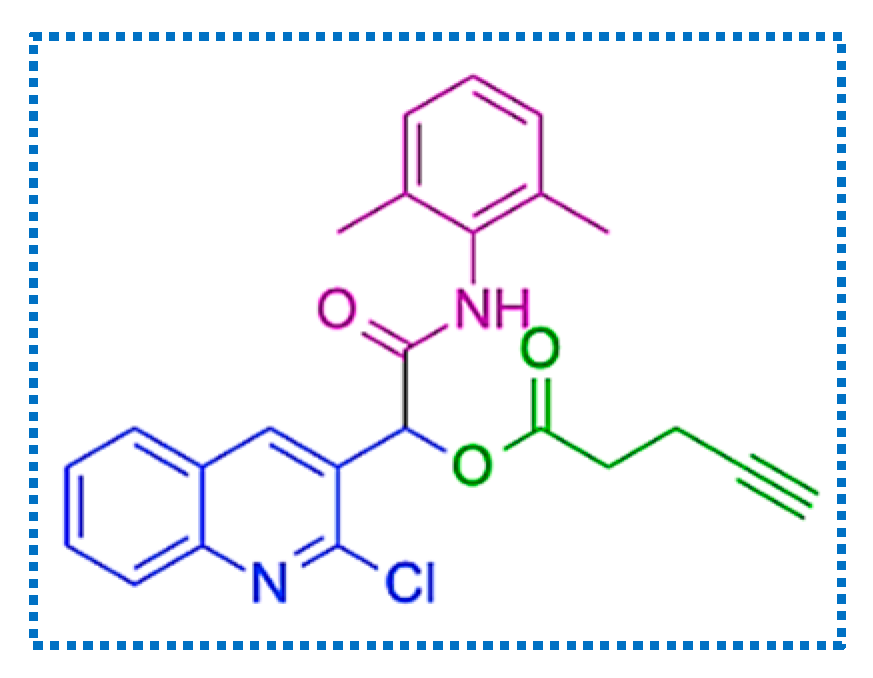
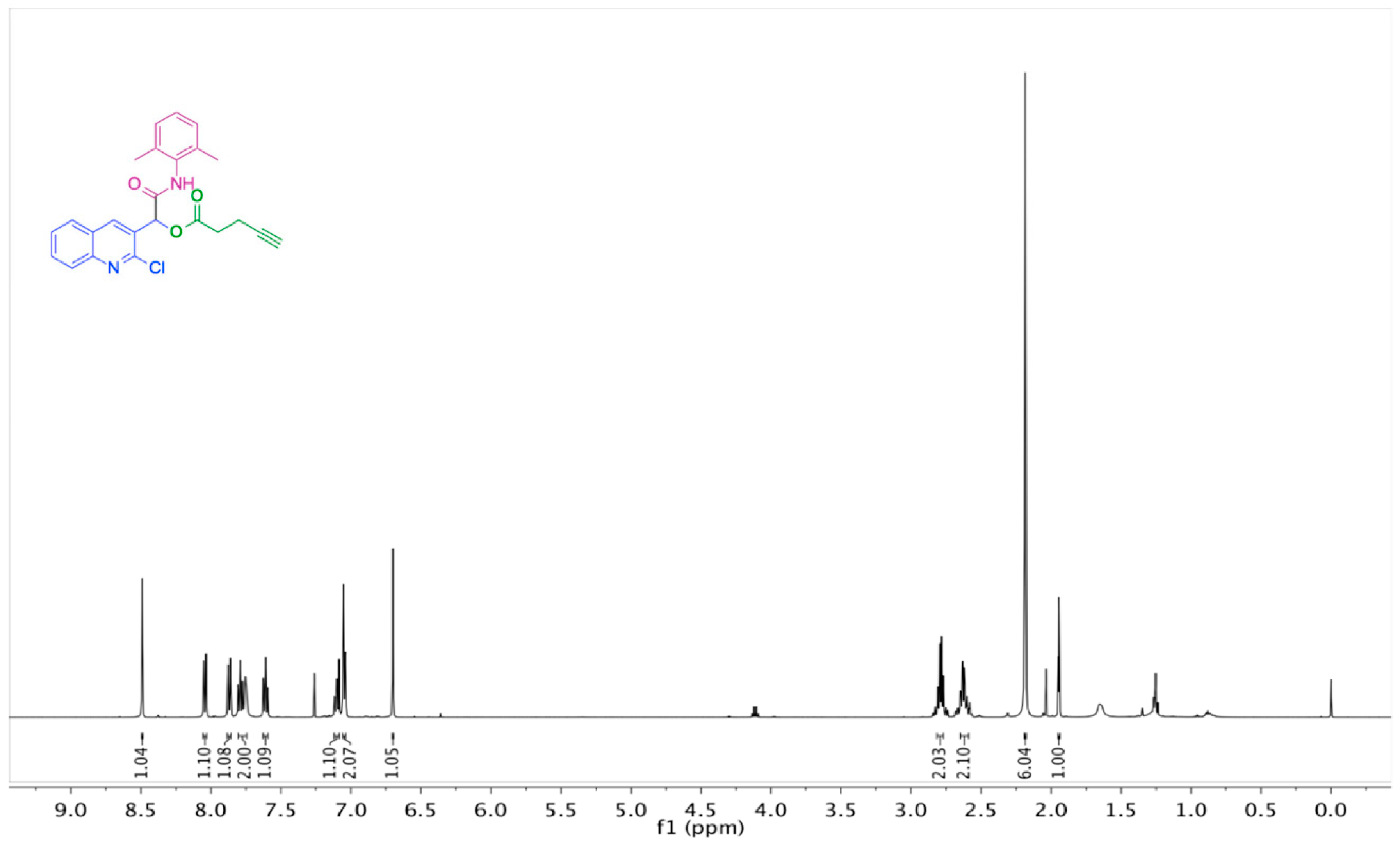
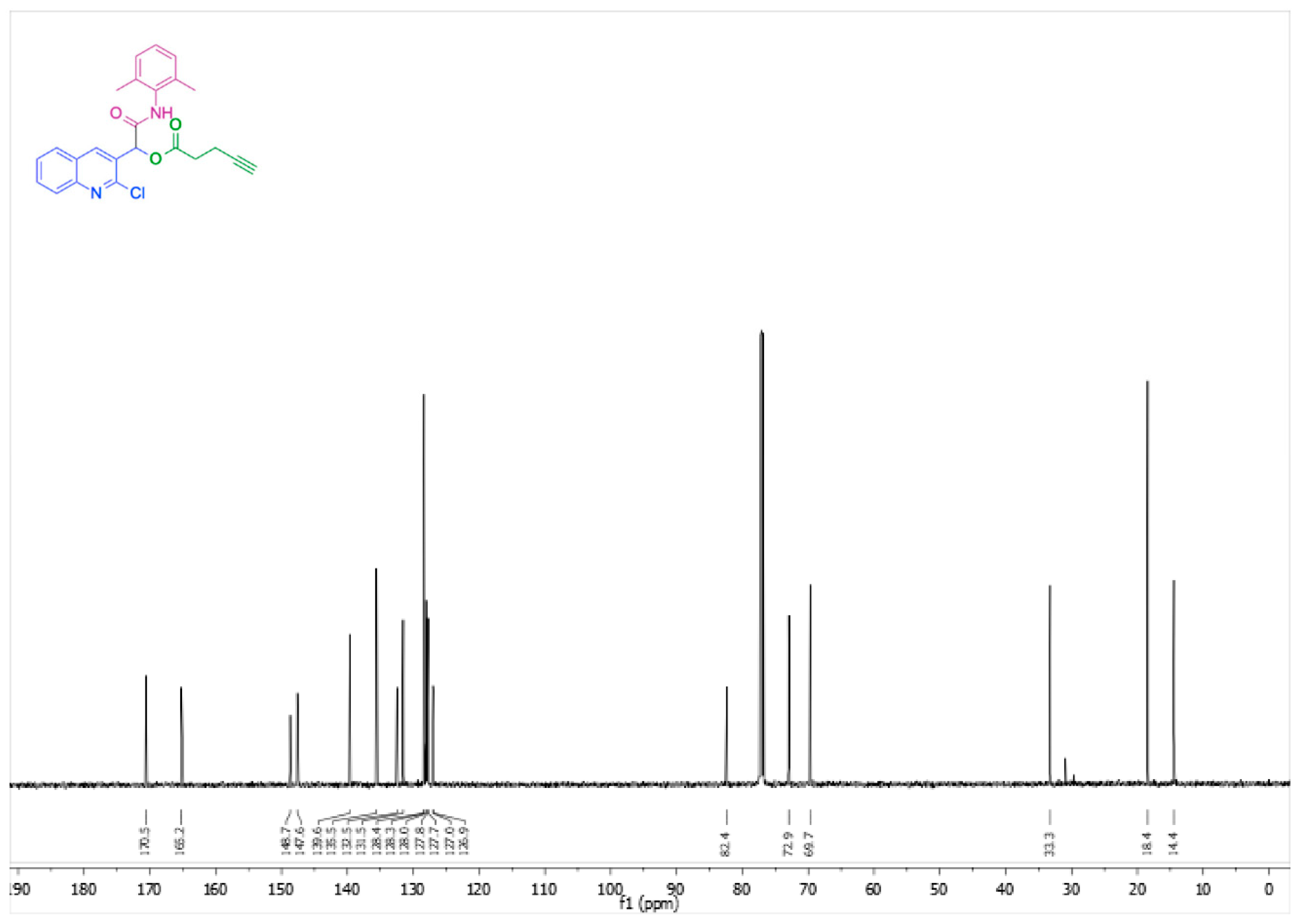
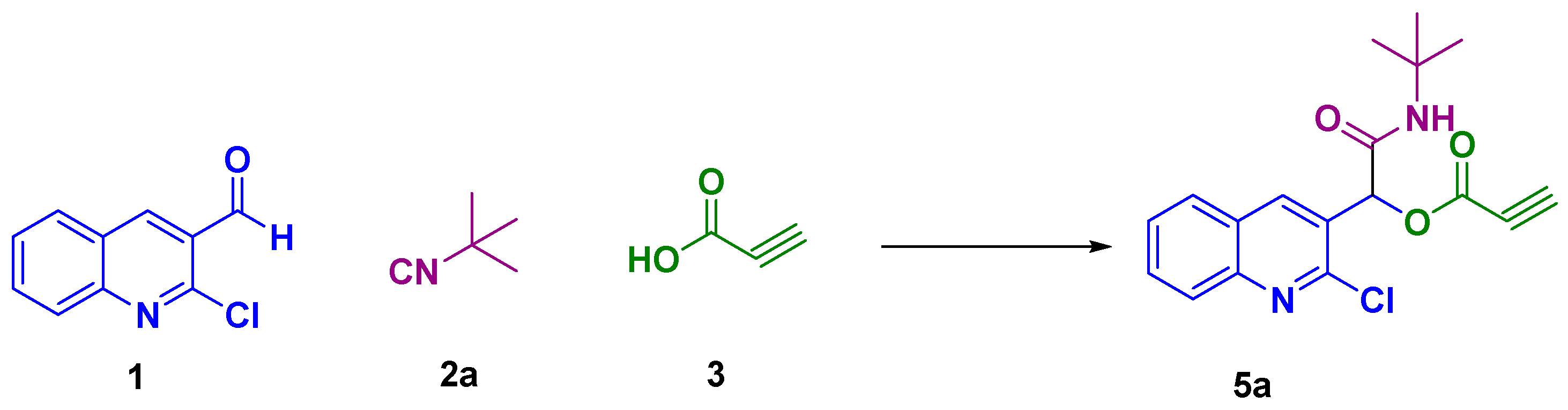
| Entry | Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | MeOH/H2O (1:1) | r.t | 8 | 17 |
| 2 | MeOH/H2O (1:1) | r.t USI | 3 | 21 |
| 3 | H2O | r.t | 24 | n.r |
| 4 | H2O | r.t USI | 3 | n.r |
| 5 | Solvent Free | r.t USI | 3 | n.r |
| 6 | Solvent Free | 60 USI | 3 | n.r |
| 7 | Surfactant (1M) | r.t USI | 3 | 12 |
| 8 | DCM/H2O (1:1) | r.t | 8 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-López, S.C.; Gámez-Montaño, R. Synthesis One Pot of Alkyne-2-Chloroquinoline via a Passerini Reaction. Proceedings 2019, 41, 76. https://doi.org/10.3390/ecsoc-23-06700
Ramírez-López SC, Gámez-Montaño R. Synthesis One Pot of Alkyne-2-Chloroquinoline via a Passerini Reaction. Proceedings. 2019; 41(1):76. https://doi.org/10.3390/ecsoc-23-06700
Chicago/Turabian StyleRamírez-López, Sandra C., and Rocío Gámez-Montaño. 2019. "Synthesis One Pot of Alkyne-2-Chloroquinoline via a Passerini Reaction" Proceedings 41, no. 1: 76. https://doi.org/10.3390/ecsoc-23-06700
APA StyleRamírez-López, S. C., & Gámez-Montaño, R. (2019). Synthesis One Pot of Alkyne-2-Chloroquinoline via a Passerini Reaction. Proceedings, 41(1), 76. https://doi.org/10.3390/ecsoc-23-06700





