Abstract
A series of six fused bis-heterocycles having imidazo[1,2-a]pyridine bound with quinoline were synthesized by microwave-assisted Groebke-Blackburn-Bienaymé reaction (GBBR) under green catalysis. The GBB products are privileged scaffolds and their synthesis is of great interest in synthetic and medicinal chemistry.
1. Introduction
Nitrogen is considered a key element with a vital role in bioactive compounds providing a noticeable range of activities [1]. Heterocycles containing nitrogen atoms possess several pharmaceutical properties. For instance, the quinoline core has received much attention in medicinal chemistry resulting from their activities e.g., anti-tuberculosis [2], antibacterial [3], antifungal [4], antimalarial [5], anti-HIV [6], and anti-inflammatory [7] activities. As a result of their pharmacological and biological properties of bioactive compounds incorporated quinoline ring system, the 2-chloroquinoline-3-carbaldehyde has been applied as an aldehyde moiety in multicomponent reactions as Ugi-4CR [8,9,10], Ugi-azide [11], and Groebke-Blackburn-Bienaymé (GBBR) [11,12,13].
Among them, bis-heterocycles have attracted much attention due to their numerous applications in many fields, such as organic synthesis, optics, materials, and polymer sciences, agrochemistry, and mainly in medicinal chemistry [14]. The research towards finding novel applications is supported on the basic principle, a union makes force [15]. Two heterocycles can be suitably placed to construct complex products having potential new or enhanced known properties.
Imidazo[1,2-a]pyridine core is present in a lot of drugs, useful in various treatments of brain diseases and CNS-related disorders. For example, zolpidem is the most prescribed drug for insomnia [15]. The GBBR is one of the highest important classes of isocyanide-based multicomponent reactions (I-MCRs) and the most efficient methods for the synthesis of fused imidazole analogs. GBBR usually requires a solvent and catalyst. In this context, GBBR methodologies using various green catalysts, such as Lewis acids, Bronsted acids, solid-supported organic bases and inorganic salts have been reported [16]. It is highlighted that the reported methodologies have some drawbacks such as high temperature, low yields, expensive catalyst and non-greener solvents.
Microwave irradiation (MW) plays a central role in synthetic organic chemistry mainly to decrease reaction times. Herein the goal is to develop an efficient protocol for the synthesis of new heterocyclic scaffolds containing imidazole and quinoline cores under ecofriendly conditions. MW offers several benefits over conventional methods including short reaction time and yield enhancements.
Foroumadi and co-workers reported in 2015 the synthesis of imidazo[1,2-a]pyridine in moderate yields (82–93%) via a GBBR using NH4Cl in stoichiometric quantities and hard conditions (Scheme 1) [17]. Our methodology has some advantages, for example, the synthetic process worked well under green catalyst. Besides short reaction times and MW energy source.
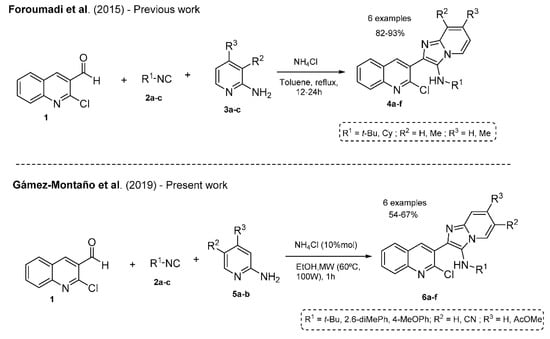
Scheme 1.
Previous works related to the synthesis of imidazo[1,2-a]pyridines analogs.
The principal objective in our research group during the last years has been to develop versatile and efficient I-MCR-based methodologies to synthesize a series of novel unsymmetrical bis-heterocycles containing privileged nucleus in medicinal chemistry [10,11,12,18].
As a part of our research program in the development of green or eco-friendly IMCR strategies herein, we describe the one-pot synthesis of new unsymmetrical bis-heterocycles via the GBBR under mild conditions. The target molecules containing two different complex heterocycles such as imidazo[1,2-a]pyridine bound with quinoline moiety.
2. Results and Discussion
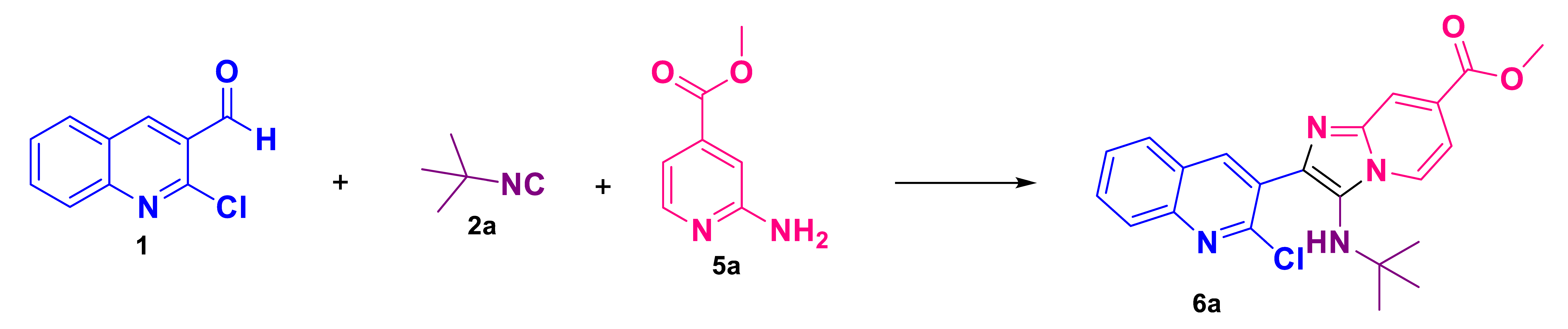
Following our interests in the efficient synthesis of bis-heterocycles containing frameworks of interest in medicinal chemistry, our work consists of the synthesis of six new analogs of 2-chloroquinoline imidazo[1,2-a]pyridine which were synthesized in moderate to good yields (54–67%) via GBBR under mild green conditions (Scheme 2).
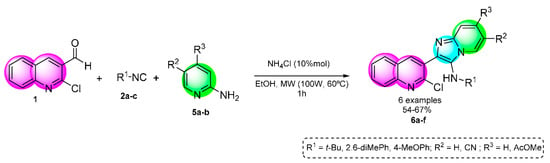
Scheme 2.
Strategy for the synthesis of analogs of 2-chloroquinoline imidazo[1,2-a]pyridine.
In order to find the optimum conditions for the GBBR involved in the synthetic strategy toward unsymmetrical bound-type bis-heterocycles 6a, 2-chloro-3-formylquinoline (1) was reacted sequentially with one equivalent of methyl-2-aminopyridine-4-carboxylate (5a), tertbutyl isocyanide (2a) which were selected as the model reaction (Table 1). According to our main line of research, green solvents, moderate temperatures, and green catalysts were studied to optimize the reaction conditions. Firstly, we performed the GBBR at room temperature in water (Table 1, entry 1) but the starting materials remained completely unconsumed. When the reaction was carried out in ethanol without catalyst at r.t (Table 1, entry 3), the product 6a was obtained in 13% yield. Due to the low yields obtained, we decided to use a catalyst and we decided to try a reaction using NH4Cl in 10% mol which is a green, inexpensive and easily available catalyst. In the literature, there are many reports of the use of NH4Cl in GBBR in stoichiometric amounts and longer reaction times were required. In 2018, we reported for the first time of NH4Cl in GBBR in a catalytic amount [18]. The TLC of the reaction with NH4Cl revelated that the product yield was increased. Under MW at 60 °C in EtOH and NH4Cl, the yield was better than USI 54 and 42% of 6a respectively. The course of the reaction was monitored by TLC and characterized by 1H y 13C NMR.

Table 1.
Reaction optimizing conditions 6a.
Using optimized conditions, a series of six new 2-chloroquinoline imidazo[1,2-a]pyridine were synthesized (shown in Scheme 3). The versatility of the developed methodology was examined using different isocyanides such as aryl and alkyl (2a–c) and two different aminoazines with withdrawing groups. The respective products 6a–f were obtained in moderate to good yields (54–67%).
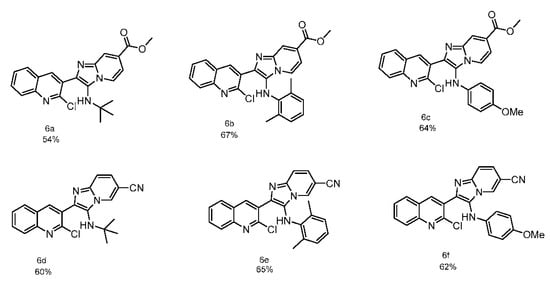
Scheme 3.
Substrate scope.
3. Experimental Section
General Information: 1H and 13C NMR spectra were acquired on a 500 MHz spectrometer. The solvent for NMR samples was CDCl3. Chemical shifts are reported in parts per million (δ/ppm). Internal reference for NMR spectra is tetramethylsilane at 0.00 ppm. Coupling constants are reported in Hertz (J/Hz). Multiplicities of the signals are reported using the standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR spectra were analyzed using the MestreNova software version 10.0.1-14719 (Mestrelab Research, S.L., Santiago de Compostela, Spain). The reaction progress was monitored by TLC and the spots were visualized under UV light (254 or 365 nm). Flash column chromatography was performed using silica gel (230–400 mesh) and mixtures in different proportions of hexanes with ethyl acetate as mobile phase. Chemical names and drawings were obtained using the ChemBioDraw Ultra 13.0.2.3020 software package (PerkinElmer Inc., Waltham, MA, USA).
General method: 2-Chloroquinoline-3-carboxaldehyde 1 (0.365 mmol, 1.0 equiv), 2-aminopyridine derivatives with withdrawing groups 5a–b (0.365 mmol, 1 equiv), isocyanide 2a–c (0.365 mmol, 1 equiv) and NH4Cl 10%mol in MeOH (1M) were placed in a 10 mL vial equipped with a magnetic stirring bar. The reaction mixture was MW-heated at 60 °C (100 W) for 1h. Then, the solvent was removed to dryness and the crude was purified by silica-gel column chromatography using mixtures of hexanes with ethyl acetate (1/1; v/v) to afford products 6a–f.
Spectral data: 2-(2-Chloroquinolin-3-yl)-3-((4-methoxyphenyl)amino)imidazo[1,2-a]pyridine-6-carbonitrile (6f): yellow solid (97.0 mg, 62%); Rf = 0.32 (Hexanes-EtOAc = 1/1 v/v); 1H NMR (500 MHz, CDCl3) δ 8.36 (s, 1 H), 8.25 (s, 1 H), 8.0−7.98 (m, 1 H), 7.78−7.68 (m, 1 H), 7.57−7.54 (m, 1 H), 7.34−7.31 (m, 1 H), 6.73−6.70 (m, 2 H), 6.45−6.43 (m, 2 H), 3.70 (s, 3 H); 13C NMR (126 MHz, CDCl3) δ 154.3, 153.1, 148.6, 147.4, 141.4, 140.9, 140.2, 137.5, 136.7, 131.3, 129.5, 128.3, 127.9, 127.6, 126.8, 126.2, 124.7, 123.2, 119.0, 116.5, 115.4,115.3,108.0, 98.9, 56.6. (Figure 1, Figure 2 and Figure 3).
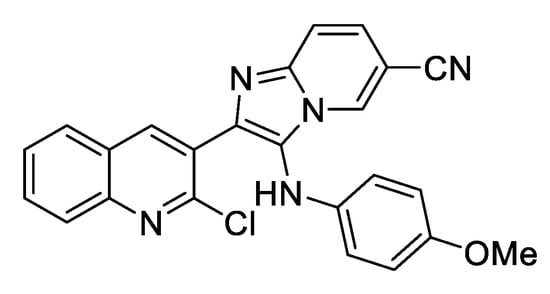
Figure 1.
Compound 6f.
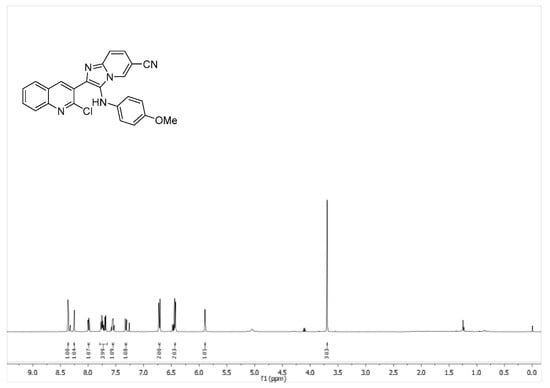
Figure 2.
1H NMR spectrum of compound 6f.
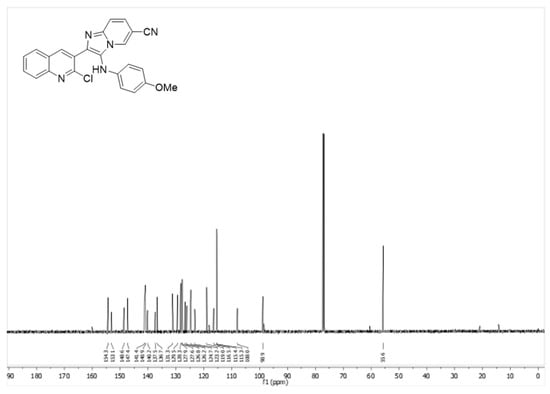
Figure 3.
13C NMR spectrum of compound 5c.
4. Conclusions
In conclusions, herein we described GBBR protocol under green catalyst for the synthesis of bis-heterocycles containing privileged nucleus in medicinal chemistry.
Additionally, this strategy has advantages in comparison with previous works due to the strategy herein described takes place under mild and green conditions.
Author Contributions
All authors contributed equally to this work.
Acknowledgments
R.G.-M. is grateful for CONACYT-México (CB-2016-285622) and DAIP-UG (CIIC 154/2019) for financial support, S.C.R.-L. acknowledges CONACYT-México for scholarship (701343/582679), the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímícas y Estructura Molecular (CONACYT-México, Project: 123732) for the instrumentation time provided.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.F. Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem. Rev. 2018, 108, 264–287. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg. Med. Chem. Lett. 2014, 24, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Sah, P.; Garg, S.P.; Nautiyal, S.R. Some new biologically active quinoline analogues. Indian J. Heterocycl. Chem. 1998, 7, 201–204. [Google Scholar]
- Vandekerckhove, S.; D’hooghe, M. Quinoline-based antimalarial hybrid compounds. Bioorg. Med. Chem. 2015, 23, 5098–5119. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brahmbhatt, K.G.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and anti-HIV activity of alkylated quinoline 2,4-diols. Bioorg. Med. Chem. 2010, 18, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, S.A.H.; Abd El-Samii, Z.K.; Osman, N.A.; Lashine, J.; Kamel, M.A.; Thabet, H.K. Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg. Chem. 2015, 58, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Asthana, M.; Sharma, N.; Singh, R.M. Densely functionalized 1,2-dihydrobenzo[b][1,6]naphthyridines: One-pot synthesis via sequential Ugi and Heck reactions. Tetrahedron 2014, 70, 7996–8003. [Google Scholar] [CrossRef]
- Asthana, M.; Kumar, R.; Gupta, T.; Singh, R.M. Facile synthesis of functionalized 1H-pyrrolo[2,3-b]quinolines via Ugi four-component reaction followed by Cu-catalyzed aryl-amide, C–N bond coupling. Tetrahedron Lett. 2015, 56, 907–912. [Google Scholar] [CrossRef]
- Ghandi, M.; Zarezadeh, N.; Abbasi, A. One-pot synthesis of spiropyrroloquinoline-isoindolinone and their aza-analogs via the Ugi-4CR/metal-free intramolecular bis-annulation process. Org. Biomol. Chem. 2015, 13, 8211–8220. [Google Scholar] [CrossRef] [PubMed]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Álamo, M.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2-a]pyridin Tetrazolo[1,5-a]quinolines. J. Org. Chem. 2016, 81, 10576–10583. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.G.; Islas-Jácome, A.; Rentería-Gómez, A.; Conejo, A.S.; Basavanag, U.M.V.; Wrobel, K.; Gámez-Montaño, R. Synthesis of unsymmetrical bis-heterocycles containing the imidazo[2,1-b]thiazole framework and their benzo[d]fused analogues by an acid-free Groebke–Blackburn–Bienaymé reaction. Tetrahedron Lett. 2016, 57, 3556–3560. [Google Scholar] [CrossRef]
- Claudio-Catalán, M.Á.; Pharande, S.G.; Quezada-Soto, A.; Kishore, K.G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis-Heterocycles Synthesis via Groebke–Blackburn–Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Premakumari, C.; Muralikrishna, A.; Padmaja, A.; Padmavathi, V.; Park, S.J.; Kim, T.-J.; Reddy, G.D. Synthesis, antimicrobial and anticancer activities of amido sulfonamido methane linked bis heterocycles. Arab. J. Chem. 2014, 7, 385–395. [Google Scholar] [CrossRef]
- Murru, S.; Nefzi, A. Combinatorial Synthesis of Oxazol-Thiazole Bis-Heterocyclic Compounds. ACS Comb. Sci. 2014, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.S.; Keating, G.M. ; Zolpidem. CNS Drugs 2005, 19, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienaymé multicomponent reaction: Emerging chemistry for drug discovery. Mol. Divers 2016, 20, 233−254. [Google Scholar] [CrossRef] [PubMed]
- Dianat, S.; Mahdavi, M.; Moghimi, S.; Mouradzadegun, A.; Shafiee, A.; Foroumadi, A. Combinated isocyanide-based multi-component Ullmann-type reaction:an efficient access to novel nitrogen-containing pentacyclic compounds. Mol. Divers 2015, 19, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Mahanandaiah, K.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo[1,2-a]pyridines via Groebke-Blackburn-Bienaymé reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).