Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition †
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods and Materials
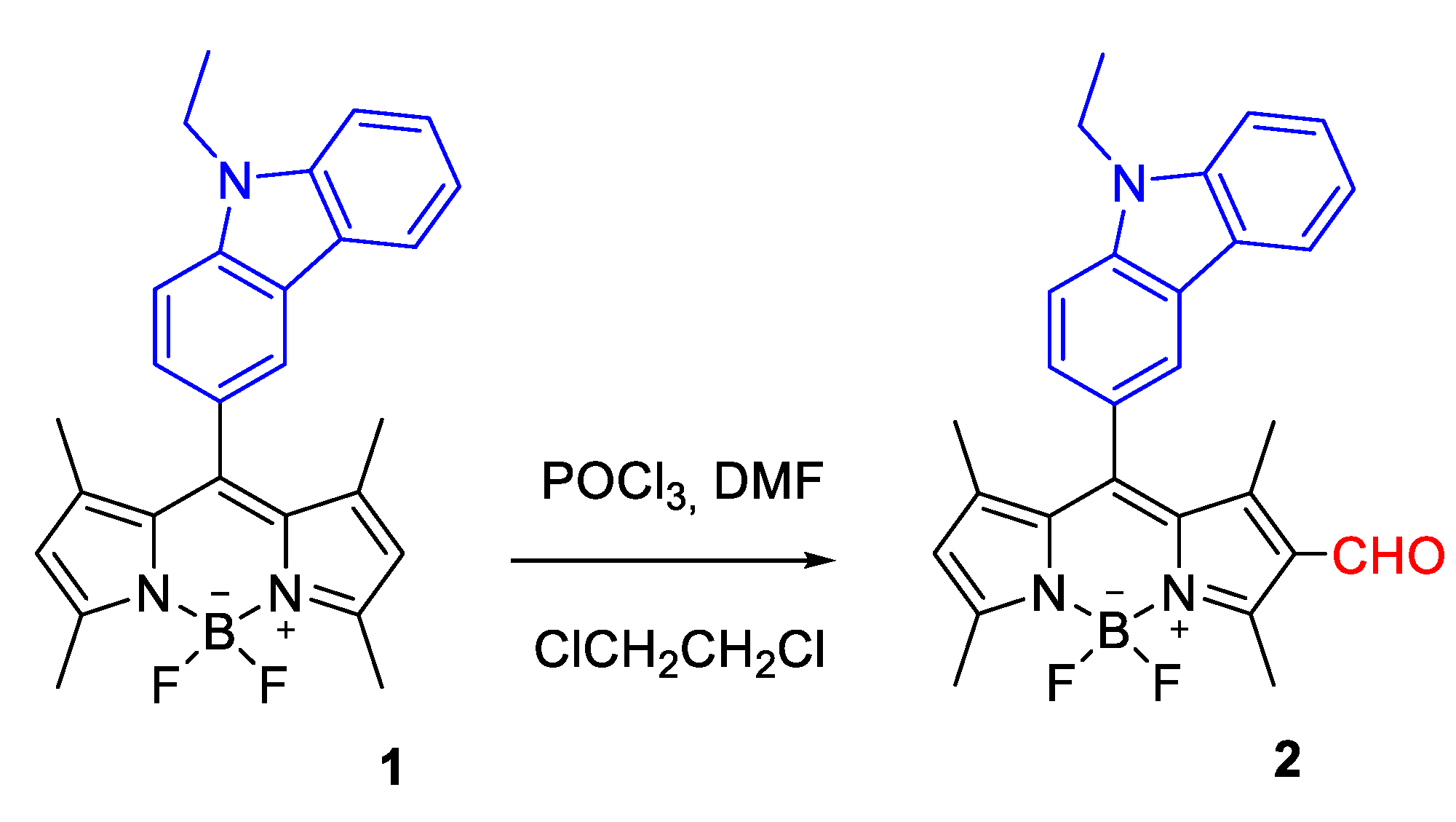
2.2. Synthesis of BODPY Derivative 2
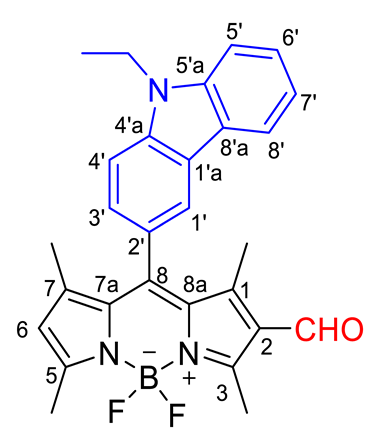
- 1H RMN (400 MHz, CDCl3): δ = 1.37 (s, 3H, CH3-7), 1.37 (t, J = 7.2 Hz, 3H, N(CH2CH3), 1.61 (s, 3H, CH3-1), 2.65 (s, 3H, CH3-5), 2.86 (s, 3H, CH3-3), 4.45 (q, J = 7.2 Hz, 2H, N(CH2CH3), 6.15 (s, 1H, H-6), 7.27–7.35 (m, 2H, H-3′ e H-4′), 7.52–7.58 (m, 3H, H-6′, H-7′ e H-8′), 8.00 (s, 1H, H-1′), 8.08 (d, J = 7.6 Hz, 1H, H-5′), 10.01 (s, 1H, CHO) ppm.
- 13C NMR (100.6 MHz, CDCl3): δ = 11.85, 13.04, 13.8, 15.06, 15.14, 37.87, 108.94, 109.16, 119.50, 119.88, 120.62, 122.46, 123.59, 123.75, 124.15, 124.91, 126.30, 126.56, 130.65, 134.88, 135.82, 140.18, 143.13, 145.15, 147.52, 156.22, 161.09, 185.96 ppm.
- MS (ESI) m/z (%): 471 ([M + 2]+•, 31), 470 ([M + 1]+•, 100), 469 ([M]+•, 23), 437 (9), 393 (8), 349 (6), 305 (3), 242 (52); HRMS (ESI) m/z: [M + 1]+• for C28H27BF2N3O calcd. 470.2210; found 470.2225.
2.3. Chemosensing Studies of BODIPY Derivative 2 and Spectrophotometric Titration
3. Results and Discussion
3.1. Synthesis of BODIPY Derivative 2
3.2. Photophysical Characterization of BODIPY Derivative 2
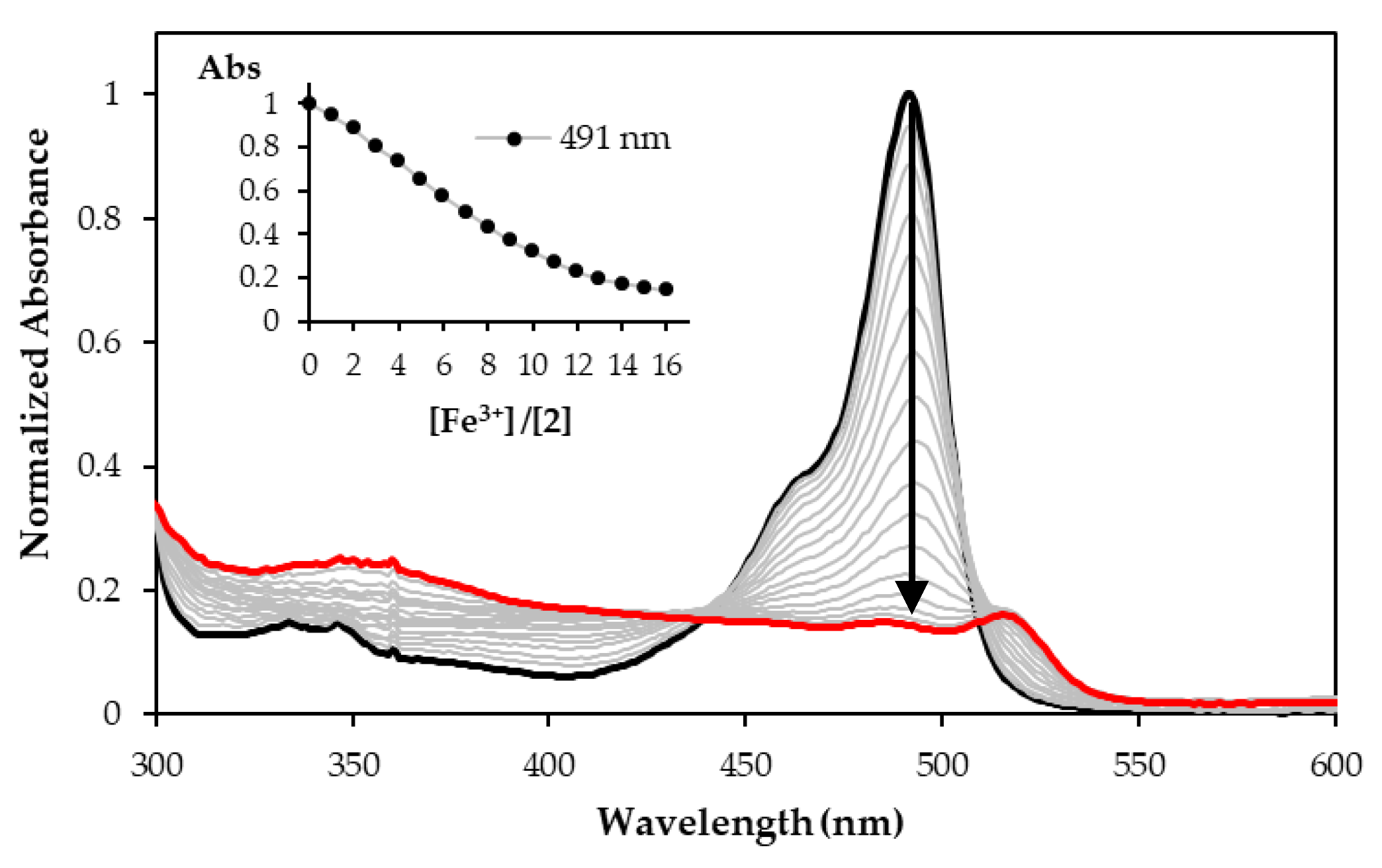
3.3. Chemosensing Studies of BODIPY Derivative 2 and Spectrophotometric Titration
4. Conclusions
Funding
Conflicts of Interest
References
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2015, 2015, 6577–6595. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, Oxidative Stress and Neurodegeneration: A Focus on Iron, Manganese and Mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric Metal Ion Sensors—A Comprehensive Review of the Years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A Dual Channel Sulphur-containing Macrocycle Functionalised BODIPY Probe for the Detection of Hg(II) in Mixed Aqueous Solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New Fluoroionophores for Metal Cations Based on Benzo[d]Oxazol-5-yl-Alanine Bearing Pyrrole and Imidazole. Dyes Pigments 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Selective colorimetric and fluorimetric detection of cyanide in aqueous solution using novel heterocyclic imidazo-anthraquinones. Sens. Actuators B Chem. 2014, 191, 791–799. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M.; (University of Minho, Braga, Portugal). Personal communication, 2019.
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. Rev. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition. Proceedings 2019, 41, 40. https://doi.org/10.3390/ecsoc-23-06625
Gonçalves RCR, Pinto SCS, Costa SPG, Raposo MMM. Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition. Proceedings. 2019; 41(1):40. https://doi.org/10.3390/ecsoc-23-06625
Chicago/Turabian StyleGonçalves, Raquel C. R., Sónia C. S. Pinto, Susana P. G. Costa, and M. Manuela M. Raposo. 2019. "Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition" Proceedings 41, no. 1: 40. https://doi.org/10.3390/ecsoc-23-06625
APA StyleGonçalves, R. C. R., Pinto, S. C. S., Costa, S. P. G., & Raposo, M. M. M. (2019). Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition. Proceedings, 41(1), 40. https://doi.org/10.3390/ecsoc-23-06625





