1. Introduction
Biomass is a complex natural renewable material with enormous chemical variability and great potential application. Biomass is defined as matter originating from living plants, including tree stems, branches, leaves as well as residues from agricultural harvesting and processing of seeds or fruits [
1]. The valorization of natural compounds obtained from biomass is a topic of growing study in many areas of chemical sciences.
Rye flour is a product of biomass and it is used extensively for making a range of bread, as a filler for sauces, soups, and custard powder, but it is also used in the glue, match and plastics industries.
The rye protein, when hydrated, does not form gluten because the proportion of the protein that is soluble is much larger in rye than in wheat (up to 80% soluble in rye sourdough as compared with 10% soluble protein in wheat dough), and because the high content of pentosans inhibits the formation of gluten [
2]. Pentosans are hemicellulose, the soluble fraction of polysaccharides. Conversely, the pentosans and starch in rye are much more important than in wheat. The pentosans, which comprise 4%–7% of rye flour, and the starch have an important water-binding function [
3].
In this research work, the extraction of proteins and polysaccharides from rye flour using an isotonic aqueous solution was performed. Then, with these isolates the synthesis of nanoparticles using fluorescein as a model drug were evaluated. These biopolymer nanoparticles have shown to be interesting as active compound release systems with different applications.
2. Materials and Methods
2.1. Materials
Rye (Secale cereale) flour was obtained from commercial source. All reagents were of analytical grade and used without purification.
2.2. Polysaccharide and Protein Extractions
Polysaccharides and proteins (PP) were extracted from 40 g of rye flour with 250 mL of 0.9 w% NaCl aqueous solution at pH 7.0 by stirring at 500 RPM for 12 h at ambient temperature. Then, the mixture was centrifuged at 3500 RPM for 20 min. The supernatant solution was separated and maintained at 4 °C overnight. As a result of the maceration of solution at 4 °C, two liquid phases were obtained: An upper clear phase and a lower viscous phase.
For this study, the upper phase was used and characterized.
2.3. Determination of Total Dry Residue
A sample of 10 mL of the upper phase was accurately weighed on a previously dry and heavy container. The sample was subsequently evaporated in an oven at 105 °C until completely dry. Finally, the container was weighed and the total dry residue of the sample was determined by weight difference based on the initial weight of the sample.
2.4. Determination of Proteins
For the quantification of proteins, the Biuret assay was used [
4]. The Biuret reagent was prepared to dissolve 2.25 g of potassium sodium tartrate, 0.75 g of cupric sulfate × 5 H
2O and 1.25 g of potassium iodide with 100 mL of 0.2 M NaOH in a volumetric flask of 250 mL. Then, the solution was diluted at 250 mL with distilled water. A sample of 1.25 mL of the upper phase was transferred quantitatively in a volumetric flask of 10 mL and diluted at volume with an aqueous solution of 0.9 % wt. NaCl. Then, 1 mL of this solution was taken and mixed with 4 mL of Biuret reagent and the mixture was stirred. Samples were incubated for 30 min at room temperature and after this time the absorbance of samples was tested. Measurement of absorbance was done with UV—VIS Perkin Elmer Lambda 20 spectrophotometer, at wavelength
λ = 550 nm. The calibration curve was made from a solution of ovalbumin in an aqueous solution of 0.9% wt. NaCl in the concentration range of 0.20–0.10 mg/mL and the equation describing it was: y = 0.044x + 0.095 with R
2 = 0.9986. The study was performed in duplicate.
2.5. Determination of Polysaccharides
For the quantification of polysaccharides, the DuBois assay was used [
5]. A sample of 50 μL of the upper phase was taken and volume was brought to 5 mL with distilled water. Then 1 mL of this solution was taken (in duplicate), and 500 μL of a solution of 0.5 % wt. phenol and 2.5 mL of concentrated sulfuric acid were added. It was homogenized and allowed to stand for 30 min in an ice bath. The absorbance of the samples so prepared was measured at wavelength
λ = 490 nm against the zero test. Calibration curve was made from sucrose solution in the concentration range 10–100 mg/L and the equation describing it was: y = 0.0115x + 0.0678 at R
2 = 0.9955. The study was performed in duplicate.
2.6. Preparation of Fluorescein Loaded PP Nanoparticles
The isolation of water-soluble polysaccharides and proteins (PP) corresponding to upper phase was used as matrix for preparation of nanoparticles. The PP nanoparticles were prepared by a desolvatation method [
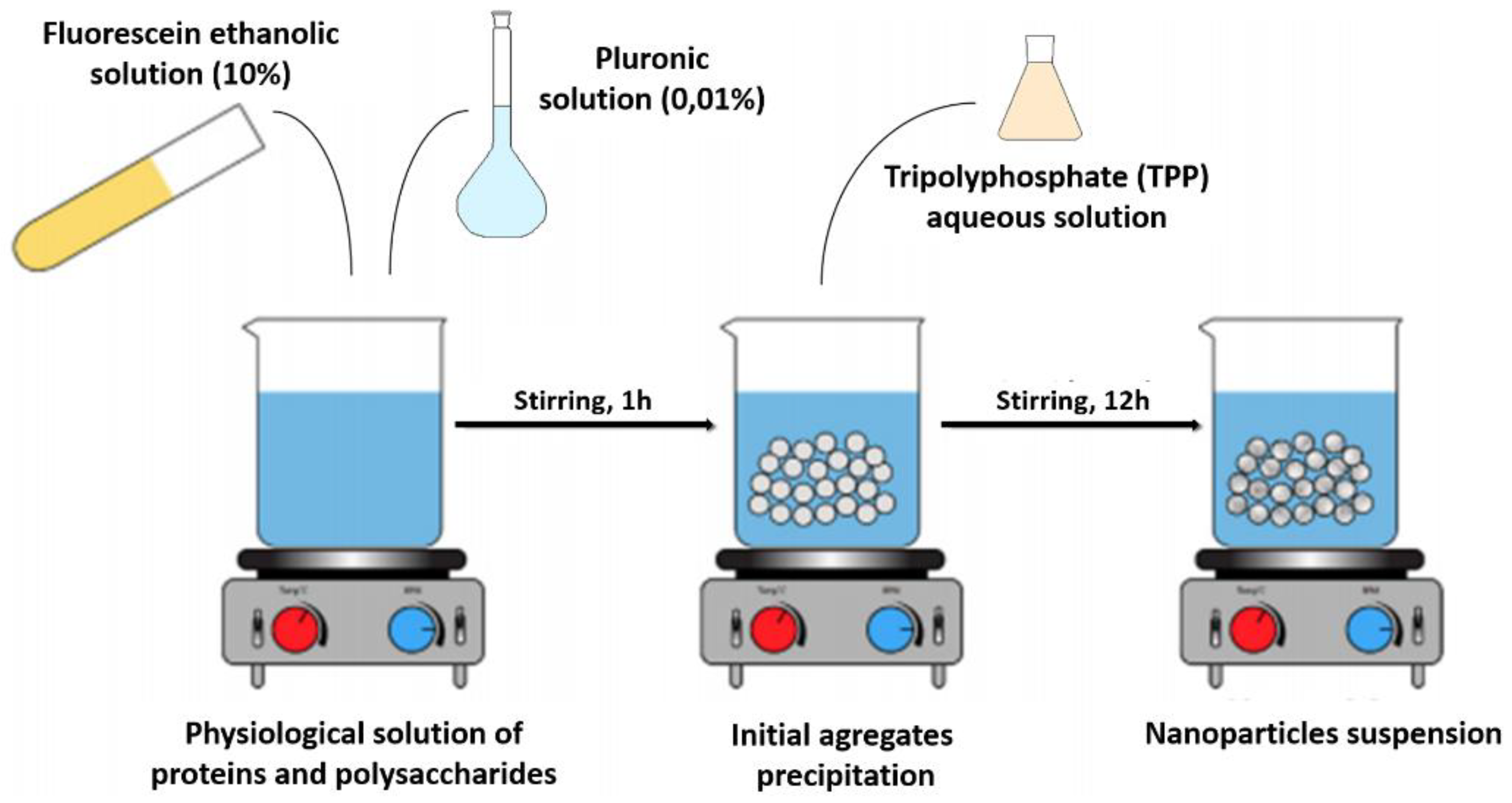
6] with modification following the scheme of
Figure 1. Typically, over 10 mL of PP solution was added dropwise 10 mL of a solution of 10 wt. % fluorescein in ethanol, followed for 10 mL of a solution of 0.1 wt. % Pluronic in ethanol. After 1 h of equilibration, a solution of 0.5 wt. sodium hexapolyphosphate (trypolifosfate) in water was added as a cross-linker. After 12 h of cross-linking reaction at room temperature, the organic solvent was evaporated and the resulting suspension was centrifuged at 3500 RPM for 20 min. The supernatant obtained after centrifugation was dried in oven under reduced pressure and stored at 4 °C.
3. Results and Discussion
3.1. Characterization of Extract of Water-Soluble Polysaccharides and Proteins of Rye Flour
The main properties obtained of the characterization of the upper phase of the isolate of water-soluble polysaccharides and proteins of rye flour are summarized in
Table 1. Similar values were reported in the literature [
7].
3.2. Characterization of Fluorescent Nanoparticles
The PP fraction, water-soluble polysaccharides and proteins of rye flour, precipitated into nanoparticles by the desolvation method as nanoprecipitates. The preparation process consisted of dispersion, desolvation, drug incorporation, crosslinking, and separation of nanoparticles.
The nanoparticles were synthesized by a rapid diffusion of polysaccharides and proteins solution with fluorescent alcohol solution, which results in surface tension at the interface between two liquids causing an increase in surface area, and leading to precipitation of PP nanoparticles. The process can take place in both the presence and absence of surfactants, but the incorporation of Pluronic increase the interaction of fluorescein with proteins and polysaccharides reducing the surface tension [
8]. The process is simple, rapid, and reproducible. The fluorescein nanoparticles are spherical, with sizes between 20 and 100 nm (determined by dynamic light scattering (DLS) technique).
4. Conclusions
The study of isolation of proteins and polysaccharides of rye flour using a isotonic solution at pH 7 and room temperature, with a relation solid:liquid of 6.25 g/mL was adequate for to obtain a liquid phase rich in polymers with concentrations of 1.09 ± 0.34 wt. % and 1.26 ± 0.05 wt. % in proteins and polysaccharides, respectively.
The aqueous extract rich in proteins and polysaccharides of rye, was subjected to a process of desolvation with sodium hexamonophosphate (TTP) to encapsulate fluorescein, obtaining spherical nanoparticles with sizes between 20 and 100 nm. These nanoparticles proved to be a suitable system to vehicle active molecules of interest.