ZnS/CuFe2O4: Magnetic Hybrid Nanocomposite to Catalyze the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives †
Abstract
:1. Introduction
2. Experimental
2.1. General
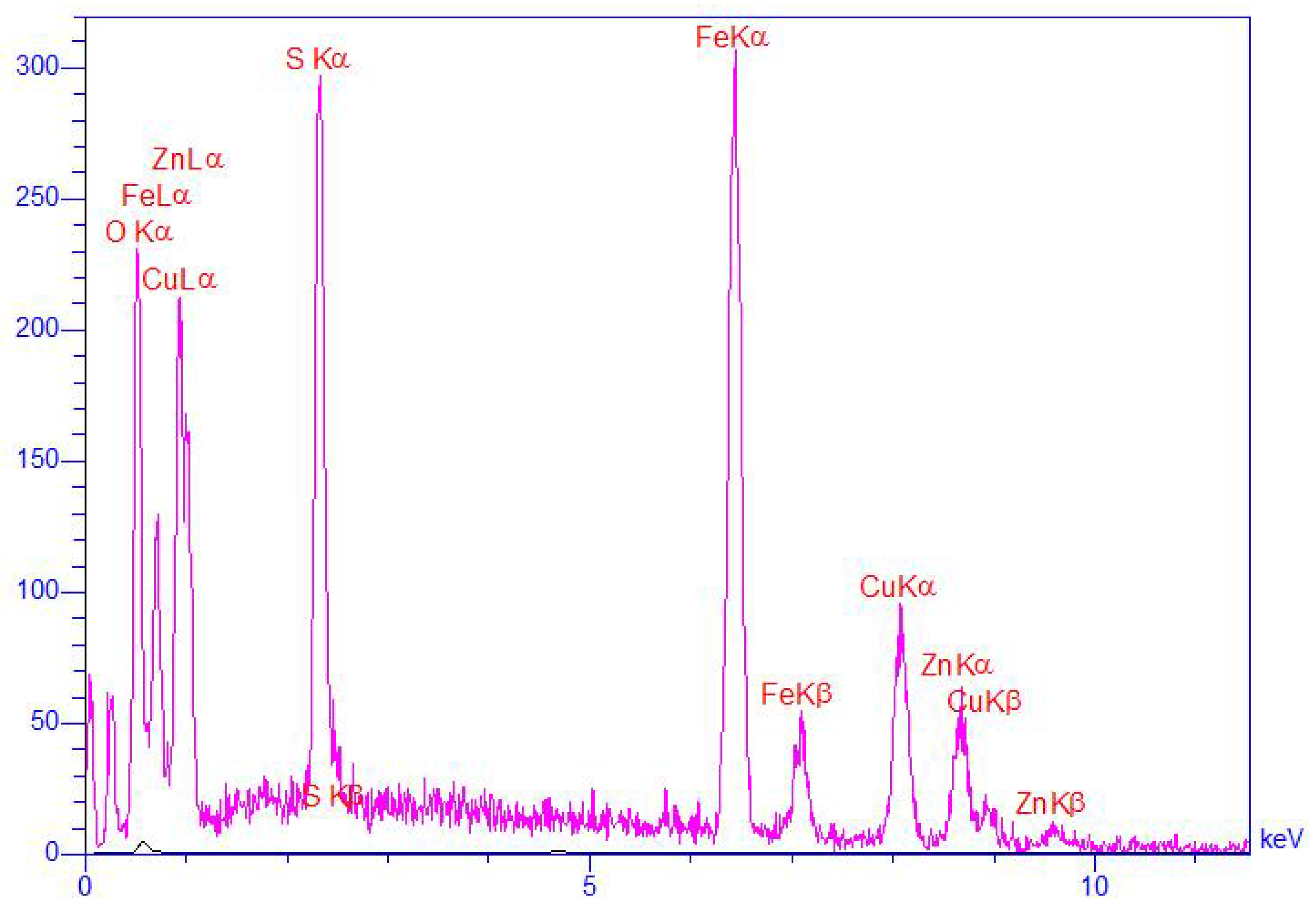
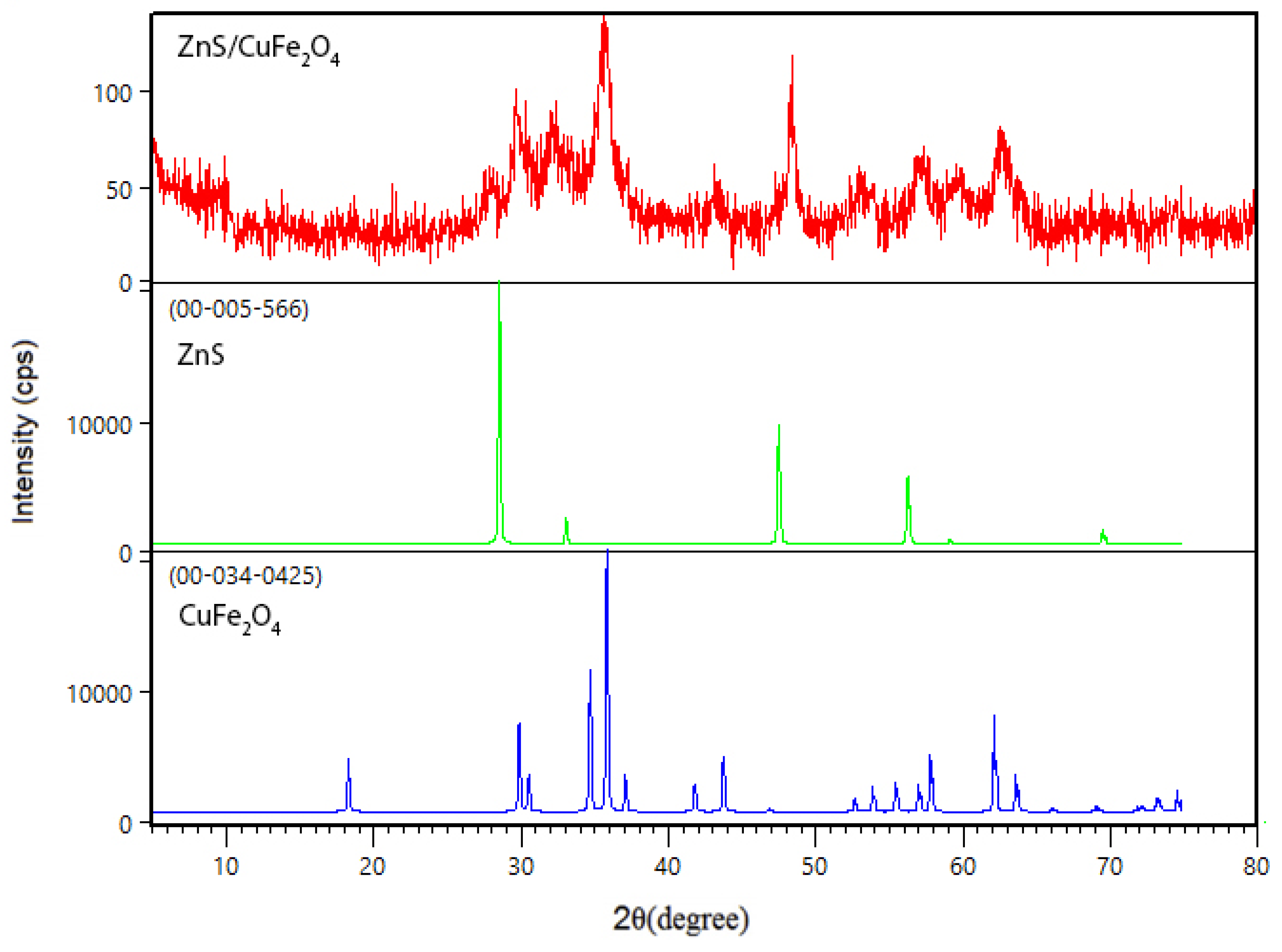
2.2. Preparation of ZnS/CuFe2O4
2.3. General Procedure for the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives
3. Results and Discussion
Catalytic Application of ZnS/CuFe2O4 in the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bavandpour, R.; Karimi-Maleh, H.; Asif, M.; Gupta, V.K.; Atar, N.; Abbasghorbani, M. Liquid phase determination of adrenaline uses a voltammetric sensor employing CuFe2O4 nanoparticles and room temperature ionic liquids. J. Mol. Liq. 2016, 213, 369–373. [Google Scholar] [CrossRef]
- Kumar, A.S.; Reddy, M.A.; Knorn, M.; Reiser, O.; Sreedhar, B. Magnetically recoverable CuFe2O4 nanoparticles: Catalyzed synthesis of aryl azides and 1,4-diaryl-1,2,3-triazoles from boronic acids in water. Eur. J. Org. Chem. 2013, 21, 4674–4680. [Google Scholar] [CrossRef]
- Yeo, K.M.; Shin, J.; Lee, I.S. Reductive dissolution of Fe3O4 facilitated by the Au domain of an Fe3O4/Au hybrid nanocrystal: Formation of a nanorattle structure composed of a hollow porous silica nanoshell and entrapped Au nanocrystal. Chem. Commun. 2010, 46, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Xu, G.; Tan, S.; Zhu, Y. A facile sol-gel strategy for the scalable synthesis of CuFe2O4 nanoparticles with enhanced infrared radiation property: Influence of the synthesis conditions. Infrared Phys. Technol. 2017, 85, 261–265. [Google Scholar] [CrossRef]
- Paramasivan, P.; Venkatesh, P. Controllable synthesis of CuFe2O4 nanostructures through simple hydrothermal method in the presence of thioglycolic acid. Phys. E 2016, 84, 258–262. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Behnam, M. Tandem oxidation process using ceric ammonium nitrate: Three-component synthesis of trisubstituted imidazoles under aerobic oxidation conditions. Synth. Commun. 2008, 39, 102–110. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Chen, C.; Li, X.; Cao, H. Ag-Catalyzed Tandem Three-Component Reaction toward the Synthesis of Multisubstituted Imidazoles. J. Org. Chem. 2017, 82, 13740–13745. [Google Scholar] [CrossRef]
- Maleki, A. Fe3O4/SiO2 nanoparticles: An efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 2012, 68, 7827–7833. [Google Scholar] [CrossRef]
- Maleki, A. One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett. 2013, 54, 2055–2059. [Google Scholar] [CrossRef]
- Maleki, A. One-pot three-component synthesis of pyrido [2′, 1′: 2, 3] imidazo [4,5-c] isoquinolines using Fe3O4@ SiO2–OSO3 H as an efficient heterogeneous nanocatalyst. RSC Adv. 2014, 4, 64169–64173. [Google Scholar] [CrossRef]
- Maleki, A. Synthesis of imidazo[1,2-a]pyridines using Fe3O4@SiO2 as an efficient nanomagnetic catalyst via a one-pot multicomponent reaction. Helv. Chim. Acta 2014, 97, 587–593. [Google Scholar] [CrossRef]
- Maleki, A. Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A. An efficient magnetic heterogeneous nanocatalyst for the synthesis of pyrazinoporphyrazine macrocycles. Polycycl. Aromat. Compd. 2018, 38, 402–409. [Google Scholar] [CrossRef]
- Maleki, A.; Ghassemi, M.; Firouzi-Haji, R. Green multicomponent synthesis of four different classes of six-membered N-containing and O-containing heterocycles catalyzed by an efficient chitosan-based magnetic bionanocomposite. Pure Appl. Chem. 2018, 90, 387–394. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Firouzi-Haji, R. Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Microporous Mesoporous Mater. 2018, 259, 46–53. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Abbasi, H. Surface modification of graphene oxide by citric acid and its application as a heterogeneous nanocatalyst in organic condensation reaction. Carbon Lett. 2018, 27, 42–49. [Google Scholar]
- Maleki, A.; Ghalavand, R.; Firouzi-Haji, R. A novel and eco-friendly o-phenylendiamine stabilized on silica-coated magnetic nanocatalyst for the synthesis of indenoquinoline derivatives under ultrasonic-assisted solvent-free conditions. Iran. J. Catal. 2018, 8, 221–229. [Google Scholar]
- Maleki, A.; Akhlaghi, E.; Paydar, R. Design, synthesis, characterization and catalytic performance of a new cellulose-based magnetic nanocomposite in the one-pot three-component synthesis of α-aminonitriles. Appl. Organometal. Chem. 2016, 30, 382–386. [Google Scholar] [CrossRef]
- Maleki, A.; Aghaei, M.; Ghamari, N. Facile synthesis of tetrahydrobenzoxanthenones via a one-pot three-component reaction using an eco-friendly and magnetized biopolymer chitosan-based heterogeneous nanocatalyst. Appl. Organometal. Chem. 2016, 30, 939–942. [Google Scholar] [CrossRef]
- Maleki, A.; Rahimi, R.; Maleki, S. Efficient oxidation and epoxidation using a chromium (VI)-based magnetic nanocomposite. Environ. Chem. Lett. 2016, 14, 195–199. [Google Scholar] [CrossRef]
- Maleki, A.; Movahed, H.; Ravaghi, P.; Kari, T. Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv. 2016, 6, 98777–98787. [Google Scholar] [CrossRef]
- Maleki, A.; Aghaei, M.; Hafizi-Atabak, H.R.; Ferdowsi, M. Ultrasonic treatment of CoFe2O4@ B2O3-SiO2 as a new hybrid magnetic composite nanostructure and catalytic application in the synthesis of dihydroquinazolinones. Ultrason. Sonochem. 2017, 37, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Shaabani, A. Green and efficient synthesis of quinoxaline derivatives via ceric ammonium nitrate promoted and in situ aerobic oxidation of α-hydroxy ketones and α-keto oximes in aqueous media. Chem. Pharm. Bull. 2008, 56, 79–81. [Google Scholar]
- Maleki, A.; Soleimani, E. One-pot three-component synthesis of 3-aminoimidazo[1,2-a]pyridines and pyrazines in the presence of silica sulfuric acid. Monatsh. Chem. 2007, 138, 73–76. [Google Scholar]


| Entry | R | Product | Yielda (%) | Mp (°C) |
|---|---|---|---|---|
| 1 | 4-Cl | 4a | 92 | 259–261 |
| 2 | 3-NO2 | 4b | 89 | 264–266 |
| 3 | 4-OMe | 4c | 83 | 228–230 |
| 4 | 3-OMe | 4d | 89 | 257–259 |
| 5 | 4-Me | 4e | 82 | 229–231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanzadeh-Afruzi, F.; Bahrami, S.; Maleki, A. ZnS/CuFe2O4: Magnetic Hybrid Nanocomposite to Catalyze the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives. Proceedings 2019, 41, 44. https://doi.org/10.3390/ecsoc-23-06654
Hassanzadeh-Afruzi F, Bahrami S, Maleki A. ZnS/CuFe2O4: Magnetic Hybrid Nanocomposite to Catalyze the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives. Proceedings. 2019; 41(1):44. https://doi.org/10.3390/ecsoc-23-06654
Chicago/Turabian StyleHassanzadeh-Afruzi, Fereshte, Shahrzad Bahrami, and Ali Maleki. 2019. "ZnS/CuFe2O4: Magnetic Hybrid Nanocomposite to Catalyze the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives" Proceedings 41, no. 1: 44. https://doi.org/10.3390/ecsoc-23-06654
APA StyleHassanzadeh-Afruzi, F., Bahrami, S., & Maleki, A. (2019). ZnS/CuFe2O4: Magnetic Hybrid Nanocomposite to Catalyze the Synthesis of 2,4,5-triaryl-1H-imidazole Derivatives. Proceedings, 41(1), 44. https://doi.org/10.3390/ecsoc-23-06654





