Performance Evaluation of Microtubular Solid Oxide Fuel Cell Prototypes at a Laboratory Scale and Identification of Needs Related to Gas Sensors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Performance Evaluation of Microtubular SOFC Prototypes
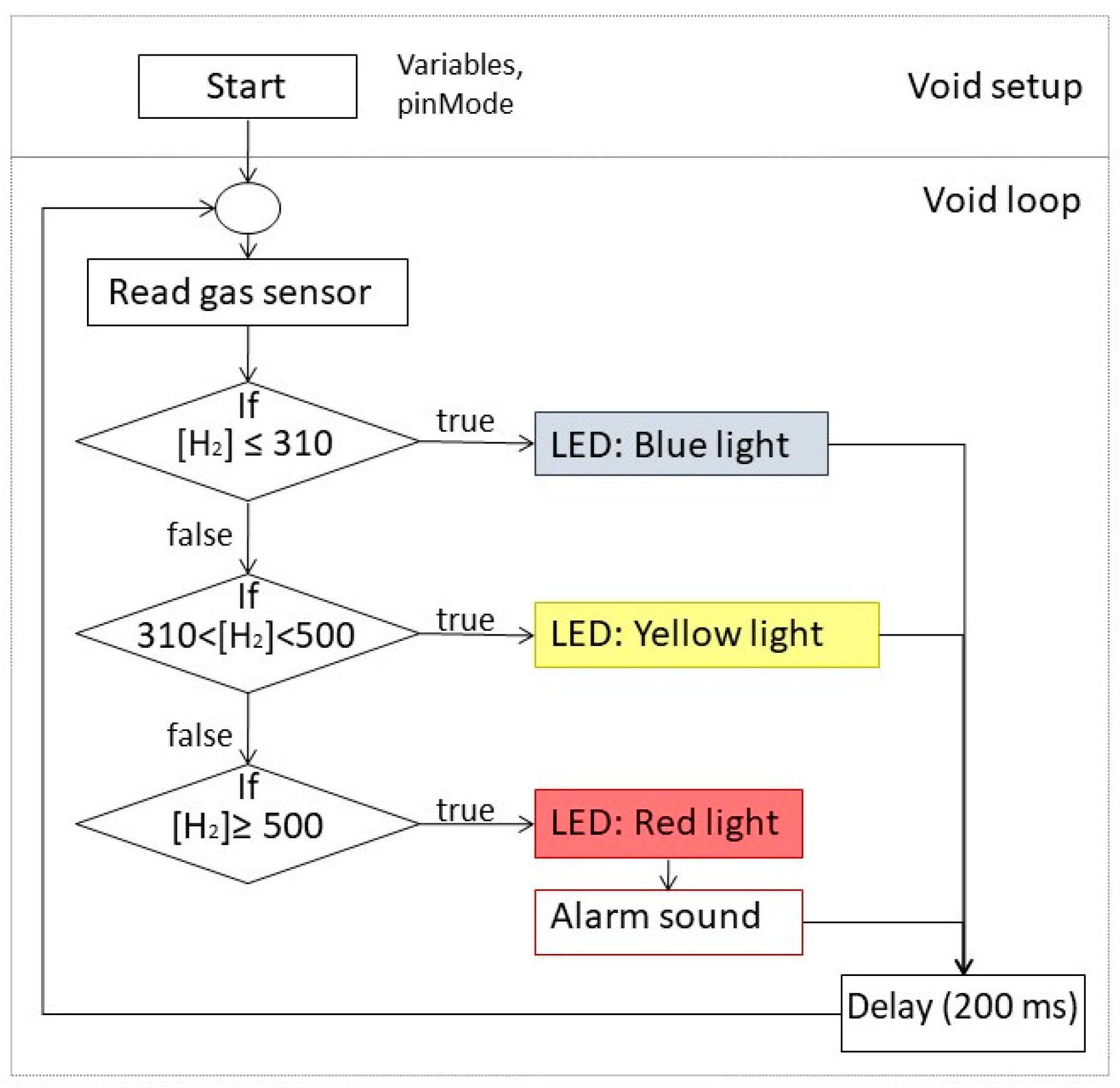
2.2. Design of a Hydrogen Detecting—Alarm System
3. Results and Discussion
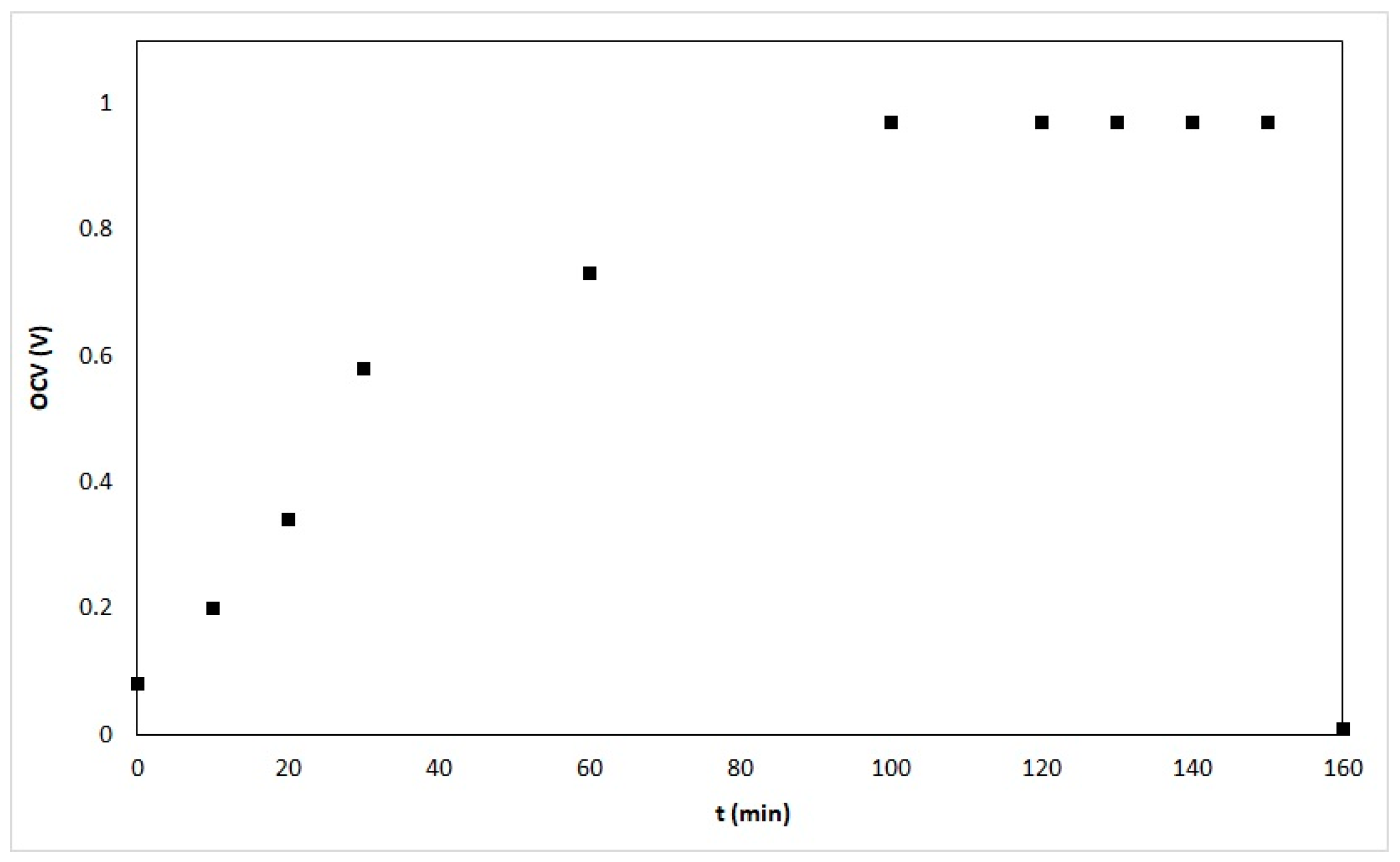
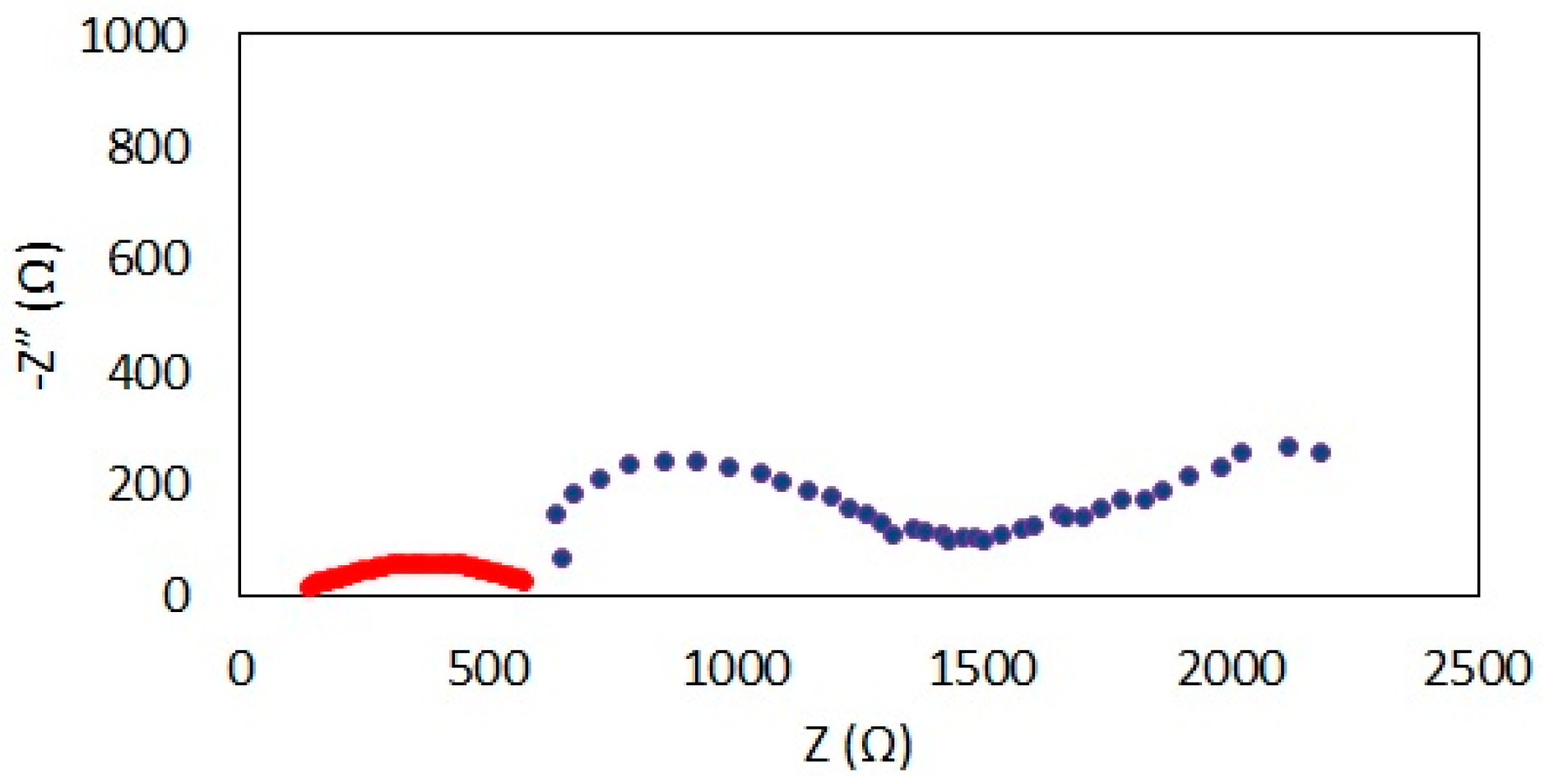
3.1. Performance Evaluation of Microtubular SOFC Prototypes
3.2. Design of a Hydrogen Detecting—Alarm System
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, X. Principles of Fuel Cell, 1st ed.; Taylor and Francis: New York, NY, USA, 2006; pp. 4–14, 21–29. ISBN 1591690226, 9781591690221. [Google Scholar]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A review on cell/stack designs for high performance solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Yang, C.; Jin, C.; Chen, F. Micro-tubular solid oxide fuel cells fabricated by phase-inversion method. Electrochem. Commun. 2010, 12, 657–660. [Google Scholar] [CrossRef]
- Howe, K.S.; Thompson, G.J.; Kendall, K. Micro-tubular solid oxide fuel cells and stacks. J. Power Sources 2011, 196, 1677–1686. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Galloway, K.V.; Yoon, J.; Sammes, N.M. Electrochemical characterizations of microtubular solid oxide fuel cells under a long-term testing at intermediate temperature operation. J. Power Sources 2011, 196, 2627–2630. [Google Scholar] [CrossRef]
- Jamil, S.M.; Othman, M.H.D.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Li, K. Recent fabrication techniques for micro-tubular solid oxide fuel cell support: A review. J. Eur. Ceram. Soc. 2015, 35, 1–22. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Yang, L.; Liu, M. Anode-supported micro-tubular SOFCs fabricated by a phase-inversion and dip-coating process. Int. J. Hydrog. Energy 2011, 36, 5604–5610. [Google Scholar] [CrossRef]
- De la Torre, R. Production of Micro-Tubular Solid Oxide Fuel Cells. Ph.D. Thesis, Department of Materials Engineering and Industrial Technologies, University of Trento, Trento, Italy, April 2011. [Google Scholar]
- De la Torre, R.; Sglavo, V.M. Fabrication of innovative compliant current collector- supported microtubular solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2012, 9, 1058–1063. [Google Scholar] [CrossRef]
- De la Torre, R.; Avila-Paredes, H.J.; Sglavo, V.M. Comparative performance analysis of anode-supported micro-tubular SOFCs with different current collection architectures. Fuel Cells 2013, 13, 729–732. [Google Scholar] [CrossRef]
- Suzuki, T.; Funahashi, Y.; Hasan, Z.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Fabrication of needle-type micro SOFCs for micro power devices. Electrochem. Commun. 2008, 10, 1563–1566. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Campana, R.; Larrea, A.; Merino, R.I.; Villareal, I.; Orera, V.M. SOFC mini-tubulares basadas en YSZ. Bol. Soc. Esp. Ceram. Vidr. 2008, 47, 189–195. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrog. Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrog. Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrog. Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Palmisano, V.; Weidner, E.; Boon-Brett, L.; Bonato, C.; Harskamp, F.; Moretto, P.; Post, M.B.; Burgess, B.; Rivkin, C.; Buttner, W.J. Selectivity and resistance to poisons of commercial hydrogen sensors. Int. J. Hydrog. Energy 2015, 40, 11740–11747. [Google Scholar] [CrossRef]
- Flammable Gas Sensor (Model; MQ-8) Manual. Available online: https://cdn.sparkfun.com/datasheets/Sensors/Biometric/MQ-8%20Ver1.3%20-%20Manual.pdf (accessed on 30 October 2017).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, C.I.R.; Rendón, C.L.F.; Avila-Paredes, H.J. Performance Evaluation of Microtubular Solid Oxide Fuel Cell Prototypes at a Laboratory Scale and Identification of Needs Related to Gas Sensors. Proceedings 2018, 2, 106. https://doi.org/10.3390/ecsa-4-04910
Villegas CIR, Rendón CLF, Avila-Paredes HJ. Performance Evaluation of Microtubular Solid Oxide Fuel Cell Prototypes at a Laboratory Scale and Identification of Needs Related to Gas Sensors. Proceedings. 2018; 2(3):106. https://doi.org/10.3390/ecsa-4-04910
Chicago/Turabian StyleVillegas, César I. Ramos, Carlos L. Fernández Rendón, and Hugo J. Avila-Paredes. 2018. "Performance Evaluation of Microtubular Solid Oxide Fuel Cell Prototypes at a Laboratory Scale and Identification of Needs Related to Gas Sensors" Proceedings 2, no. 3: 106. https://doi.org/10.3390/ecsa-4-04910
APA StyleVillegas, C. I. R., Rendón, C. L. F., & Avila-Paredes, H. J. (2018). Performance Evaluation of Microtubular Solid Oxide Fuel Cell Prototypes at a Laboratory Scale and Identification of Needs Related to Gas Sensors. Proceedings, 2(3), 106. https://doi.org/10.3390/ecsa-4-04910



