Empirical Characterization of Particle Size Distribution Spatial Dynamics for Detection of Helminth Eggs in Waste Stabilization Ponds (WSP) †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
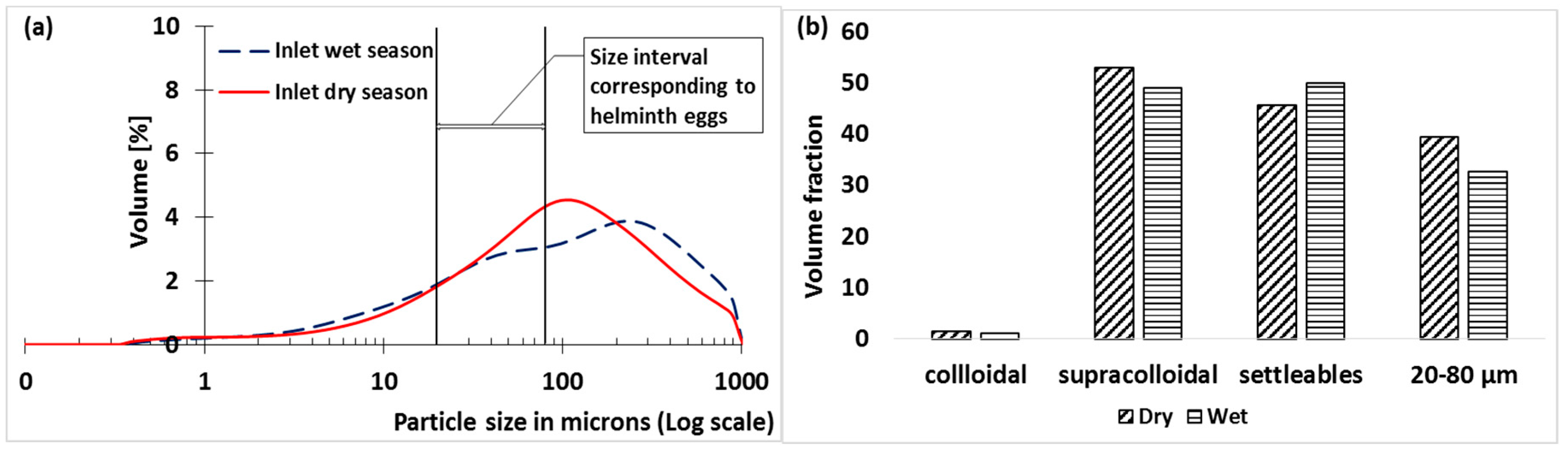
3.1. Inflow Volume Fraction Particle Size Distribution
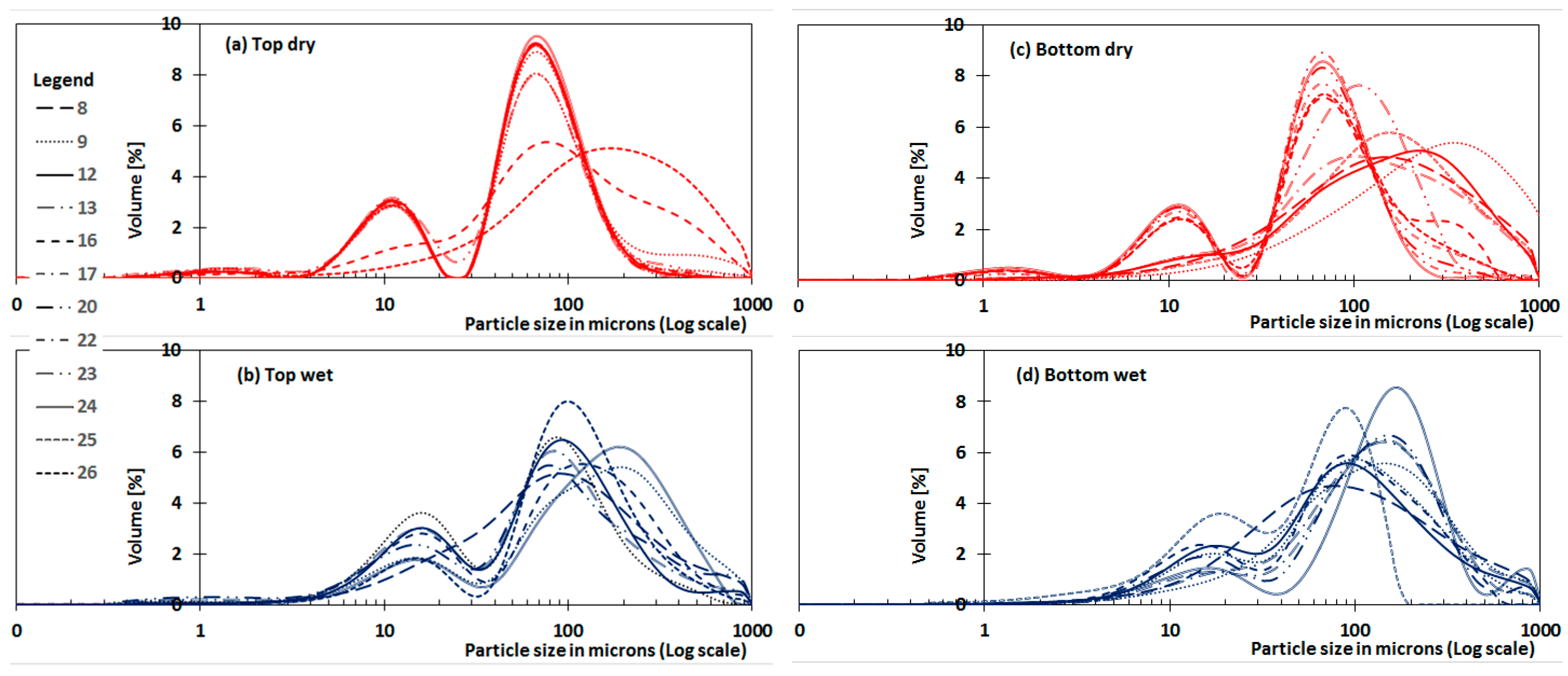
3.2. In-Pond PSD
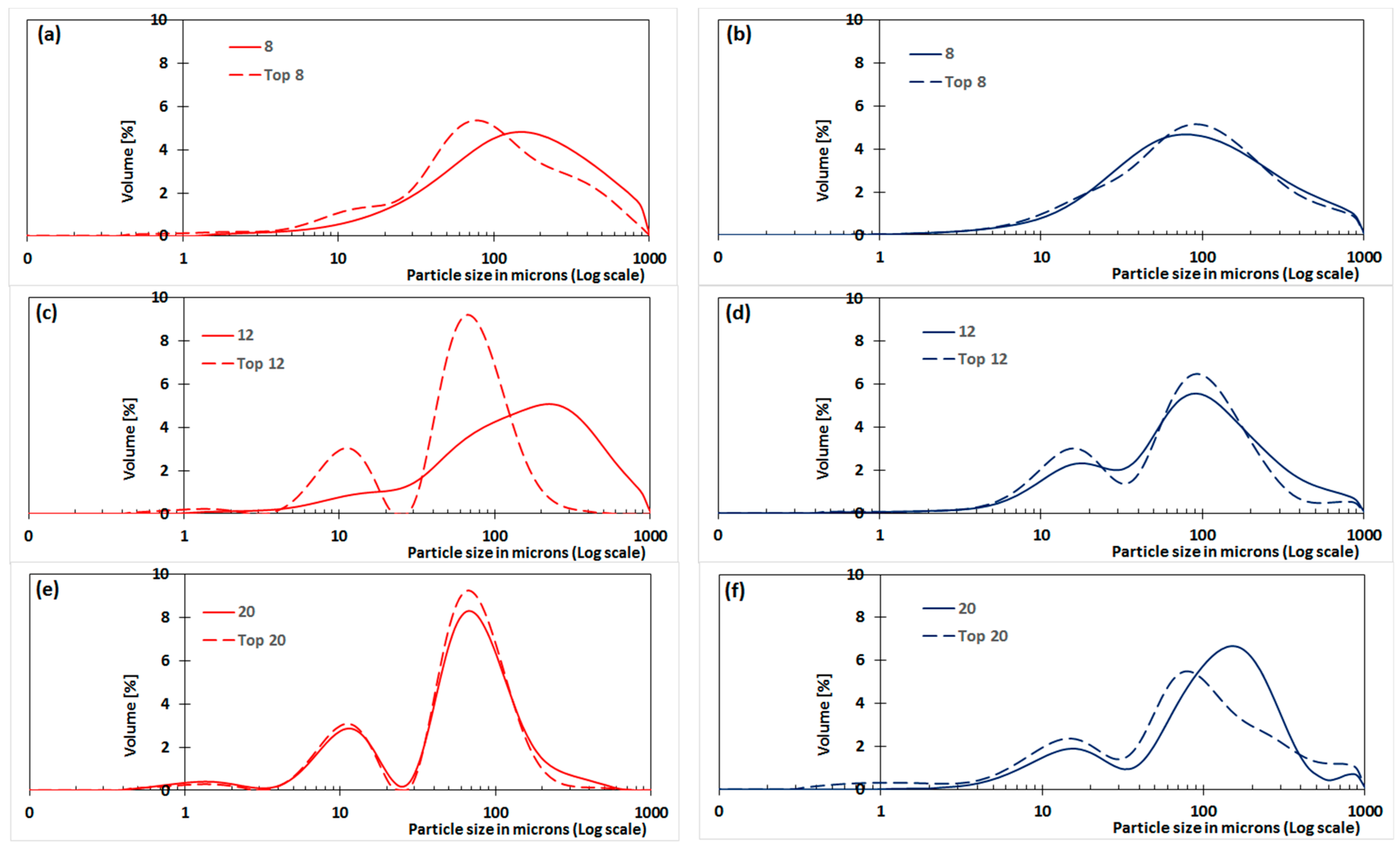
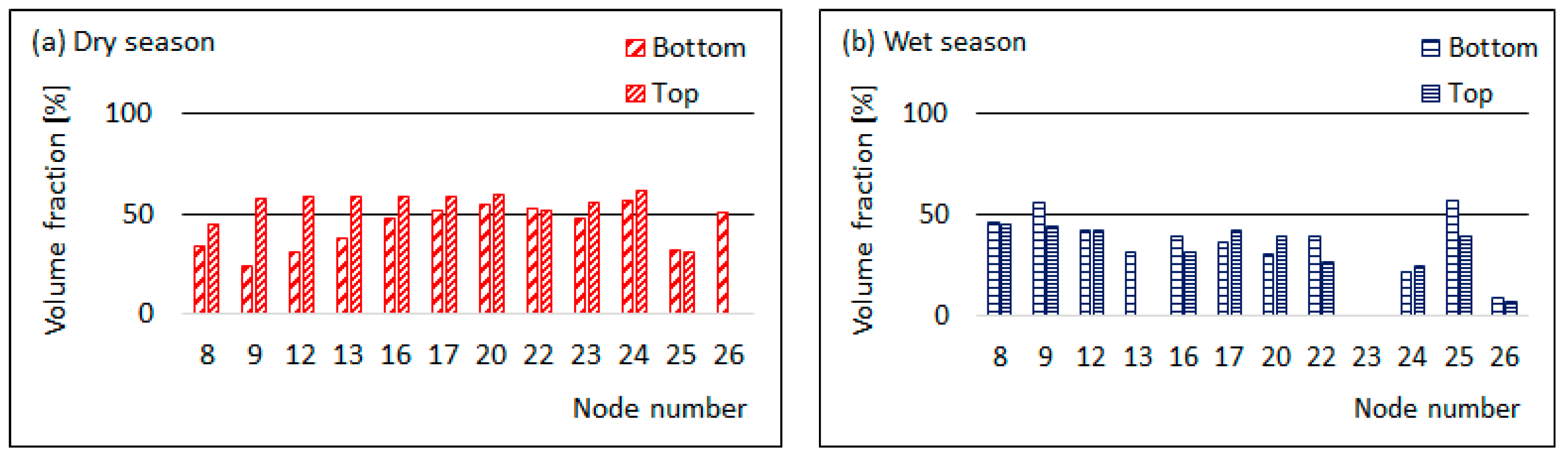
3.3. Comparison of Top and Bottom PSD
3.4. Particles with Sizes between 20 and 80 Microns
4. Conclusions
References
- Mara, D.D.; Faechem, G.A. Water- and excreta-related diseases:Unitary environmental classification. Environ. Eng. 1999, 125, 334–339. [Google Scholar] [CrossRef]
- Lloyd, B.J.; Frederick, G.L. Parasite removal by waste stabilisation pond systems and the relationship between concentrations in sewage and prevalence in the community. Water Sci. Technol. 2000, 42, 375–386. [Google Scholar] [CrossRef]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Mwanjali, G.; Kihamia, C.; Kakoko, D.V.; Lekule, F.; Ngowi, H.; Johansen, M.V.; Thamsborg, S.M.; Willingham, A.L., 3rd. Prevalence and risk factors associated with human taenia solium infections in mbozi district, mbeya region, tanzania. PLoS Negl. Trop. Dis. 2013, 7, e2102. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cisneros, B. Helminth ova control in wastewater and sludge for agricultural reuse. In Water and Health; Grabow, W.O.K., Ed.; Eolss Publishers: Oxford, UK, 2007. [Google Scholar]
- David, E.D.; Lindquist, W.D. Determination of the specific gravity of certain helminth eggs using sucrose density gradient centrifugation. J. Parasitol. 1982, 68, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Thamsborg, S.M.; Andersen, T.J.; Olsen, A.; Dalsgaard, A. Sedimentation of helminth eggs in water. Water Res. 2011, 45, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Shuval, H.I.; Adin, A.; Fattal, B.; Rawitz, E.; Yekutiel, P. Wastewater Irrigation in Developing Countries: Health Effects and Technical Solutions; World Bank: Washington, DC, USA, 1986; pp. 210–223. [Google Scholar]
- Ayres, R.M.; Stott, R.; Lee, D.L.; Mara, D.D.; Silva, S.A. Comparison of techniques for the enumeration of human parasitic helminth eggs in treated wastewater. Environ. Technol. 1991, 12, 617–623. [Google Scholar] [CrossRef]
- Stott, R.; May, E.; Mara, D. Parasite removal by natural wastewater treatment systems, performance of wastewater stabilization ponds and constructed wetlands. Water Sci. Technol. 2003, 48, 97–104. [Google Scholar] [CrossRef] [PubMed]
- von Sperling, M.; Chernicharo, C.A.L.; Soares, A.M.E.; Zerbini, A.M. Evaluation and modelling of helminth eggs removal in baffled and unbaffled ponds treating anaerobic effluent. Water Sci. Technol. 2003, 48, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ayres, R.M.; Alabaster, G.P.; Mara, D.D.; Lee, D.L. A design equation for human intestinal nematode egg removal in waste stabilization ponds. Water Res. 1992, 26, 863–865. [Google Scholar] [CrossRef]
- Shanthala, M.; Hosetti, B.B.; Stott, R. Removal helminth parasitic eggs from waste stabilization ponds at shimoga. Bioscan 2007, 2, 9–14. [Google Scholar]
- Chavez, A.; Jimenez, B.; Maya, C. Particle size distribution for microbial detection. Water Sci. Technol. 2004, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Andersen, T.J.; Dalsgaard, A.; Olsen, A.; Thamsborg, S.M. Resuspension and settling of helminth eggs in water interaction with cohesive sediments. Water Res. 2012, 46, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Keraita, B.; Olsen, A.; Boateng, O.K.; Thamsborg, S.M.; Pa’lsdo’ttir, G.R.; Dalsgaard, A. Use of moringa oleifera seed extracts to reduce helminth egg numbers and turbidity in wastewater. Water Res. 2012, 46, 3646–3656. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B.; Alma, C. Low cost technology for reliable use of mexico city’s wastewater for agricultural irrigation. J. Technol. 2002, 9, 95–108. [Google Scholar]
- Chavez, A.; Jimenez, B.; Maya, C. Particle size distribution for microbial detection. Water Sci. Technol. 2004, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.D.; Tchobanoglous, G.; Asano, T. Size distributions of particulate contaminants in wastewater and their impact on treatability. Wat. Res. 1991, 25, 911–922. [Google Scholar] [CrossRef]
- Yuan, Y.; Farnood, R.R. Strength and breakage of activated sludge flocs. Powder Technol. 2010, 199, 111–119. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izdori, F.; Semiao, A.J.C.; Perona, P. Empirical Characterization of Particle Size Distribution Spatial Dynamics for Detection of Helminth Eggs in Waste Stabilization Ponds (WSP). Proceedings 2018, 2, 178. https://doi.org/10.3390/ecws-2-04947
Izdori F, Semiao AJC, Perona P. Empirical Characterization of Particle Size Distribution Spatial Dynamics for Detection of Helminth Eggs in Waste Stabilization Ponds (WSP). Proceedings. 2018; 2(5):178. https://doi.org/10.3390/ecws-2-04947
Chicago/Turabian StyleIzdori, Fides, Andrea J. C. Semiao, and Paolo Perona. 2018. "Empirical Characterization of Particle Size Distribution Spatial Dynamics for Detection of Helminth Eggs in Waste Stabilization Ponds (WSP)" Proceedings 2, no. 5: 178. https://doi.org/10.3390/ecws-2-04947
APA StyleIzdori, F., Semiao, A. J. C., & Perona, P. (2018). Empirical Characterization of Particle Size Distribution Spatial Dynamics for Detection of Helminth Eggs in Waste Stabilization Ponds (WSP). Proceedings, 2(5), 178. https://doi.org/10.3390/ecws-2-04947



