The Impact of Hot Metal Temperature on CO2 Emissions from BOF Steelmaking †
Abstract
1. Introduction
2. System Modelling
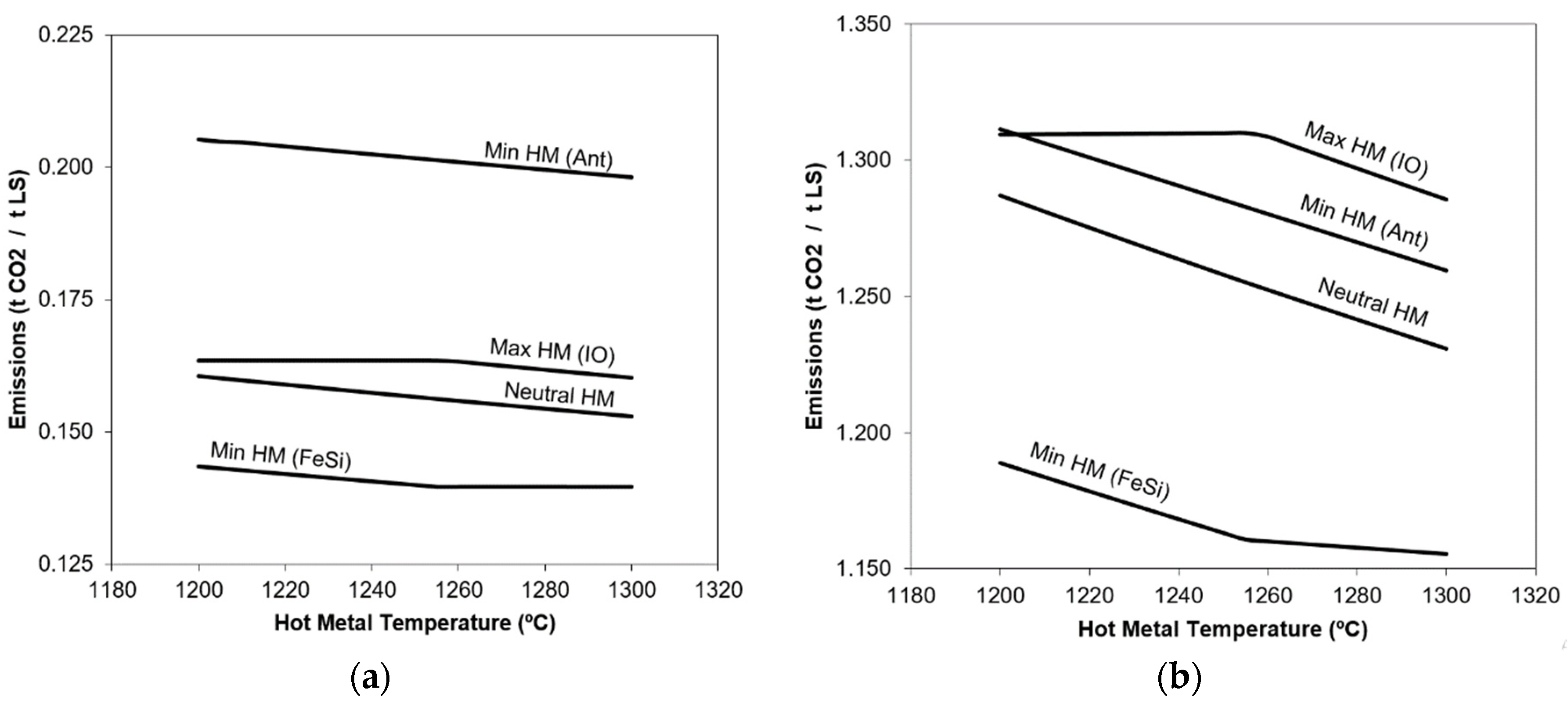
3. Results and Discussion
- Neutral energy balance, just with hot metal and scrap, without cooling or heating additions.
- Maximized hot metal, using iron ore pellets as coolant.
- Minimized hot metal, using anthracite as heating addition and producing CO2.
- Minimized hot metal, using FeSi as heating addition and generating SiO2.
4. Conclusions
Acknowledgments
References
- Steel by Topic (Life Cycle; Sustainability; Circular Economy; and Statistics). Available online: www.worldsteel.org/steel-by-topic (accessed on 1 July 2018).
- Yellishetty, M.; Mudd, G.M.; Ranjith, P.G.; Tharumarajah, A. Environmental life-cycle comparisons of steel production and recycling: Sustainability issues, problems and prospects. Environ. Sci. Policy 2011, 14, 650–663. [Google Scholar] [CrossRef]
- Ryman, C.; Larsson, M. Reduction of CO2 emissions from integrated steelmaking by optimised scrap strategies: Application of process integration models on the BF–BOF system. ISIJ Int. 2006, 12, 1752–1758. [Google Scholar] [CrossRef][Green Version]
- Barati, M. Energy intensity and greenhouse gases footprint of metallurgical processes: A continuous steelmaking case study. Energy 2010, 35, 3731–3737. [Google Scholar] [CrossRef]
- Miller, T.W.; Jimenez, J.; Sharan, A.; Goldstein, D.A. Oxygen Steelmaking Processes. In The Making, Shaping, and Treating of Steel, 11th ed.; Fruehan, R.J., Ed.; Steelmaking and Refining Volume; The AISE Steel Foundation: Pittsburgh, PA, USA, 1998; pp. 475–524. [Google Scholar]
- Mazumdar, D.; Evans, J.W. Elements of Mathematical Modeling. In Modeling of Steelmaking Processes, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2010; pp. 131–174. [Google Scholar]
- Norgate, T.; Haque, N.; Sommerville, M. Biomass as a Source of Renewable Carbon for Iron and Steelmaking. ISIJ Int. 2012, 8, 1472–1481. [Google Scholar] [CrossRef]
- González, A.L.; Fernández, F.J.; Díaz, J. Modelo de Predicción de la Temperatura de Arrabio en Acería. Master’s Thesis, University of Oviedo, Oviedo, Spain, June 2016. [Google Scholar]
- McLean, A. The Science and Technology of Steelmaking—Measurements, Models, and Manufacturing. Metall. Mater. Trans. B 2006, 37, 319–332. [Google Scholar] [CrossRef]

| Chemical Composition (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | m (t) | T (°C) | Fe | C | Si | Mn | P | O | N | Ar | CaO | MgO |
| Hot metal | m 2 | v 1 | 94.39 | 4.80 | 0.46 | 0.27 | 0.08 | |||||
| Scrap | m 2 | 25 | 99.42 | 0.05 | 0.01 | 0.50 | 0.02 | |||||
| Lime | 15 | 25 | 95 | 5 | ||||||||
| Dol. lime | 5 | 25 | 63 | 37 | ||||||||
| Iron Ore | v 1 | 25 | 70 | 30 | ||||||||
| Anthracite | v 1 | 25 | 100 | |||||||||
| FeSi | v 1 | 25 | 25 | 75 | ||||||||
| N2 | 0.15 | 25 | 100 | |||||||||
| Ar | 0.25 | 25 | 100 | |||||||||
| O2 | m 1 | 25 | 100 | |||||||||
| Steel | 300 | 1700 | 99.78 | 0.035 | 0.001 | 0.080 | 0.010 | 0.090 | ||||
| Slag | m 2 | 1700 | m 2 | m 2 | m 2 | m 2 | m 2 | m 2 | m 2 | |||
| Off-Gas | m 2 | m 2 | m 2 | m 2 | m 2 | m 2 | ||||||
| Unit | H. M. | Scrap | I. O. | Ant. | FeSi | Lime | Dol. | Ar/N2 | O2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Indirect emission [3] | (t CO2/t) | 1.1 | 0.02 | 0.18 | 0.05 | 3.9 | 1.35 | 1.35 | 0.3 | 0.3 |
| Max. amount | (t) | 280 | 80 | 4 | 4 | 2 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, J.; Fernández, F.J. The Impact of Hot Metal Temperature on CO2 Emissions from BOF Steelmaking. Proceedings 2018, 2, 1502. https://doi.org/10.3390/proceedings2231502
Díaz J, Fernández FJ. The Impact of Hot Metal Temperature on CO2 Emissions from BOF Steelmaking. Proceedings. 2018; 2(23):1502. https://doi.org/10.3390/proceedings2231502
Chicago/Turabian StyleDíaz, José, and Francisco Javier Fernández. 2018. "The Impact of Hot Metal Temperature on CO2 Emissions from BOF Steelmaking" Proceedings 2, no. 23: 1502. https://doi.org/10.3390/proceedings2231502
APA StyleDíaz, J., & Fernández, F. J. (2018). The Impact of Hot Metal Temperature on CO2 Emissions from BOF Steelmaking. Proceedings, 2(23), 1502. https://doi.org/10.3390/proceedings2231502






