1. Introduction
Nowadays, some small communities in different parts of the world and in particular in Mexico, are exposed to the intake of fluorides through water from wells of groundwater and springs [
1]. In these communities, chronic water consumption with high concentrations of fluorides trigger health problems in the population such as dental and bone fluorosis [
2]. In Mexico, in at least 6 of its states, high concentrations of fluoride in the water have been reported in different communities in the range of 3.8 to 17.7 mg·L
−1, far exceeding the limit established by the Mexican norm, NOM-127 -SSA1-1994 which establish as a maximum limit a concentration of 1.5 mg·L
−1 of fluorine in water for human consumption or as recommended by the World Health Organization (WHO) [
3] 1 mg·L
−1. As an alternative for the removal of fluorides in water are the adsorption processes with materials that are efficient, low cost and easy to implement, natural zeolites meet these particularities and their different physical and chemical properties give these materials characteristics only that can be used [
4,
5], particularly the properties of zeolites allows us to make a modification of its chemical composition surface on the ionic species present, in order to obtain a modified material that could be used as an adsorbent selective [
6,
7]. Mexico has large zeolitic deposits in several of its states and with different types of zeolites, particularly in this work a zeolite of the clinoptilolite type was used from a deposit in the place “Las Crucecitas” belonging to the municipality of Etla, in the central valley region of the state of Oaxaca in the southeast of Mexico.
2. Experimental
The sample of zeolite, natural clinoptilolite, (ZC_N) was obtained directly from the deposit and later was crushed and sieved with a No.16 mesh to obtain an average particle diameter of 1.19 mm, the crushed sample was subjected to a wash with deionized water to remove soluble material and impurities, it was subsequently left to dry at 100 °C for 24 h. In order to have an appropriate adsorbent material, the ZC_N was subjected to an ion exchange process with a 2 M NaCl solution in a ratio of 1 g of zeolite per 10 mL of solution, with constant agitation for 24 h in a rotary evaporator at room temperature. The zeolite exchanged with sodium chloride was washed with abundant deionized water to remove the chlorine ions and finally allowed to dry at 100 °C for 24 h. The sodium zeolite (ZC_Na) was exchanged with calcium or manganese ions by means of a solution of calcium nitrate or manganese of 0.01 M concentration respectively and the pH of the solution was adjusted to a value of 5, the sample was placed in agitation in a 1 g of ZC_Na with 100 mL of exchange solution in the rotavapor for 24 h, at room temperature, after the time elapsed the water was decanted and dried at 100 °C for 24 h. The conditioning with Fe was carried out by the impregnation method by precipitation, 10 g of ZC_Na and 10 mL of a 0.5 M iron nitrate solution were taken with NH4OH, afterwards it was allowed to dry at room temperature for a period of 24 h, finally to this it was washed with deionized water and allowed to dry at 100 °C.
The natural sample and materials reported in
Table 1, were characterized by different techniques such as: X-ray diffraction (XRD), in a Philip diffractometer, X’pert model, Fourier Transform Infrared Spectroscopy (FTIR), in a Varian spectrometer model Excalibur 3600, elemental chemical analysis (SEM/EDS) in a 55VP supra microscope, Carl Zeiss.
For the adsorption tests, a solution of 100 ppm of fluorine with sodium fluoride was prepared, also two standards of 10 ppm and 1 ppm were prepared, and the determination of fluorides was carried out with an ExStik selective electrode model FL700. The fluoride removal was carried out in a downflow column system with a fixed bed of 3 g of conditioned zeolite (ZC_Ca, ZC_Mn or ZC_Fe) and 30 mL of water contaminated with fluorine with two concentrations; low concentration (LC) 1.5 mg·L−1 and high concentration (HC) 10 mg·L−1 were used. The fluorine concentration was determined in the outlet water of the column after a contact period of 24 h.
3. Results and Discussion
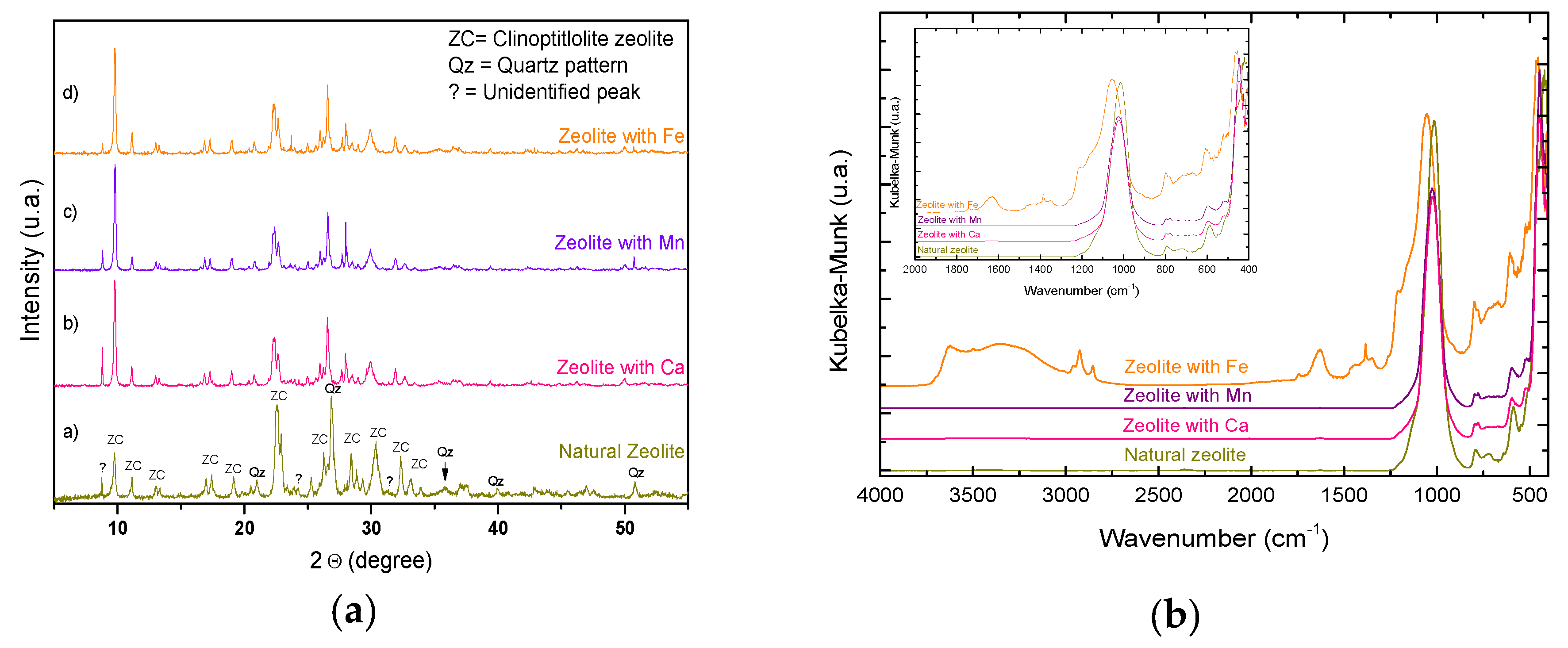
The results obtained from the natural zeolites and conditioned by the techniques of characterization by XRD, FTIR, shown in
Figure 1A that presents the predominant zeolitic phase type clinoptilolite whose main diffraction angles pattern are: 9.7°, 11.2°, 15.6°, 22.6°, 25.3°, 30.3°, 32.3° and 36.9°, being 9.7° in the 2-theta angle the one with the highest intensity corresponding to the plane (020) (PDF 01-089-7538); in
Figure 1B it is observed that the samples are slightly hydrated by bands that appear around 3600, 3400 and 1630 cm
−1 (OH group). The bands that appear around 1050, 790 and 450 cm
−1 correspond to characteristic bands of the zeolitic structure, at 1012 cm
−1 corresponds to an internal vibration of the stretching mode of the tetrahedron TO
4, and the bands at 796 and 469 cm
−1 correspond to the external vibration of the stretching mode of the OTO link. This corroborates that the structure of the zeolite is not modified or compromised with the conditioning treatment carried out. In region 780–520 cm
−1 it is observed that bands of interaction for metals are present (Ca, Mn and Fe). The elemental analysis of the samples shown in
Table 1 confirms the presence of manganese in the zeolite after conditioning and a 300% increase in calcium content and 400% increase in iron content with respect to the material in its state natural.
The
Table 2 shows the elemental composition of the natural zeolite and after being subjected to conditioning with iron and manganese and after the adsorption process. In these samples fluorine can be identified. These results show that the fluoride removed from the water is adsorbed on the zeolite. The ZC_Mn has 0.8 wt% fluorine in its composition and the ZC_Fe has a 3.31 wt%, the best removal was obtained with the zeolite modified with iron.
Table 3, shows the initial concentration of fluoride in water low concentration (LC) 1.5 mg·L
−1, high concentrations (HC) 10 mg·L
−1, the concentration of fluoride at the exit of the column as well as the percentages of removal obtained for each sample zeolite and the milligrams of fluorine adsorbed per gram of zeolite (mg·g
−1), also are reported. The natural zeolite adsorbs 0.002 mg of fluorine per gram of zeolite, for solutions with low concentrations of fluorine and for high concentrations an adsorption of 0.023 mg of fluorine per gram is achieved, which represents a maximum removal percentage of 13%. For the zeolite conditioned with calcium (ZC_Ca) obtained 53% removal in low concentration and 28% removal in high concentration. Besides, the zeolite conditioned with manganese (ZC_Mn) obtained the highest fluoride removal at low concentrations compared to its homologs, decreasing the initial concentration up to 86% and at high concentrations it obtained a 44% removal. The zeolite conditioned with iron (ZC_Fe), showed the best results reaching 73% of removal in low concentrations and up to 98% of removal in water with an initial concentration of 10 mg·L
−1.
4. Conclusions
It was determined that the natural clinoptilolite zeolite from Etla, in the central valley region of the state of Oaxaca, Mexico, has an intrinsic capacity for the removal of fluorine in water up to 0.023 mg·g−1, and the conditioning of this material with calcium, manganese or iron significantly increases this removal capacity, being the zeolite conditioning with iron, the best material because the results showed that increasing more than 80% the removal capacity of the zeolite in relation to its natural state. This means that even having water with concentrations of 10 mg·L−1 this system is able to reduce this concentration well below the limit established by the Mexican norm and the limit from WHO.
This system, column with fixed bed of natural adsorbent conditioned with Fe, result in a conventional filter that does not require complex specifications in its implementation, so it is a good option for use in the water treatment in marginalized areas of Mexico.