1. Introduction
One of the actions to achieve a reduction in CO
2 emissions is the introduction of combustion gas separation techniques, through using membrane technology [
1]. Zeolites have a significantly large, uniform porosity system, excellent thermal and chemical stability, so they are particularly promising as membranes capable of separating gases at industrial level [
2], like CO
2 from combustion gases [
3]. The gas separation properties in membranes depend on: the material (permeability, separation factors), the structure of the membrane and the thickness, the membrane wall and the design of the module and the system [
4]. After separation and capture, CO
2 can be used partially for commercial purposes or it can be stored, isolating it from the atmosphere thereby reducing its concentration in it [
5]. Carbonation involves the formation of solid carbonates, a reaction between carbon dioxide and alkaline/alkaline earth oxides [
6]. Considering these aspects, this study presents the development and evaluation of CO
2 separation technology from a mixture of post-combustion gases through a zeolitic membrane and its capture of CO
2 by carbonation.
2. Materials and Methods
A tubular silicalite-1 membrane was hydrothermally synthesized, then subjected to permeation and gas separation studies of a CO2/N2 mixture performing in a stainless steel permeation module in the 25 to 200 °C range temperature and 30 psi pressure of the feed gases: the CO2/N2 mixture was fed in 1:1 volumetric ratio. The composition of the gas flow was analyzed by means of a gas chromatograph (Agilent 6890) with an HP-PLOTQ column connected in line, with helium as the carrier gas. The synthesis gel was prepared with Degussa 200 aerosil, as a silica source, 1M tetrapropylammonium hydroxide (TPAOH) Aldrich, as structure directing agent and deionized water. The gel was allowed to mature for 72 h with constant agitation. The synthesis of the silicalite-1 layer was carried out on a commercial monotubular ceramic support of γ-alumina (Pall). The 15 cm long tubular support was waterproofed at both ends (1.5 cm each end). The synthesis of the silicalite-1 layer was carried out on a commercial monotubular ceramic support of γ-alumina (Pall). The 15 cm long tubular support was waterproofed at both ends (1.5 cm each end). The synthesis was carried out at 170 °C and autogenous pressure, for 72 h. This procedure was repeated 4 times over to ensure formation of an homogenous, defect-free silicalite-1 layer on the inner wall of the support. The membrane was characterized by elemental analysis through a SEM/EDS equipment (Carl Zeiss, model supra PV55, fitted with an Oxford detector for Energy Dispersive Spectroscopy Analysis (EDS)) and by X-ray diffraction (XRD) (in Philips X’Pert equipment). The recovered CO2 was captured by inorganic media promoting the formation of carbonates. The CO2 was fed to a reactor with Sr(OH)2, Ba(OH)2 or Mg(NO3)2 0.06M alkaline solutions at room temperature and normal atmospheric pressure (≈25–30 °C and 585 mmHg Mexico City, Mexico), with the CO2 fed at a flow of 15.17 mL/min. A Na(OH) 0.1 M solution was added to the Mg(NO3)2 solution in order to obtain Mg(OH)2, for further CO2 carbonation reactions. After reaction the solids were filtered out, then dried at 80 °C, and characterized by elemental analysis, as stated.
3. Results and Discussion
3.1. Characterization of membrane
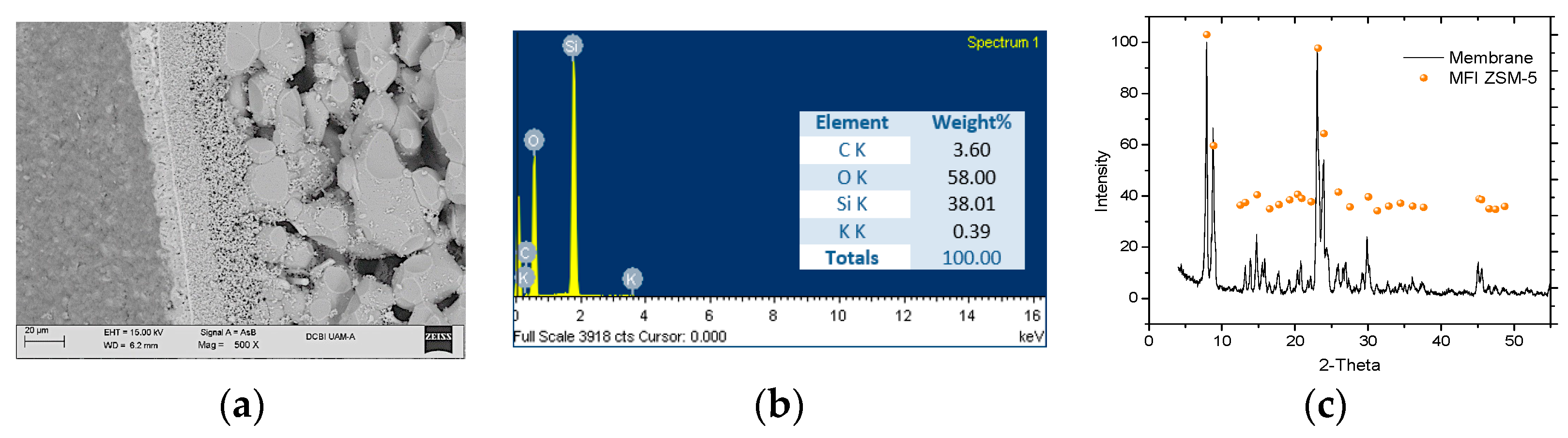
Figure 1a shows a membrane cross section SEM micrograph where the growth of the zeolite crystals can be observed on the inner support surface used in the synthesis; energy dispersive spectroscopy analysis is also presented (
Figure 1b) which yielded a composition: 58 wt% oxygen and 38.01 wt% silicon, as major elements.
Figure 1c shows the XRD diffraction pattern of the synthesized zeolite crystals compared to the zeolite MFI reference standard, obtained from the Zeolite Structures Database [
7]. In the XRD diffraction pattern, the similarity in the peaks intensity and their location in the 2θ angle can be observed.
3.2. Results of permeation and gases separation
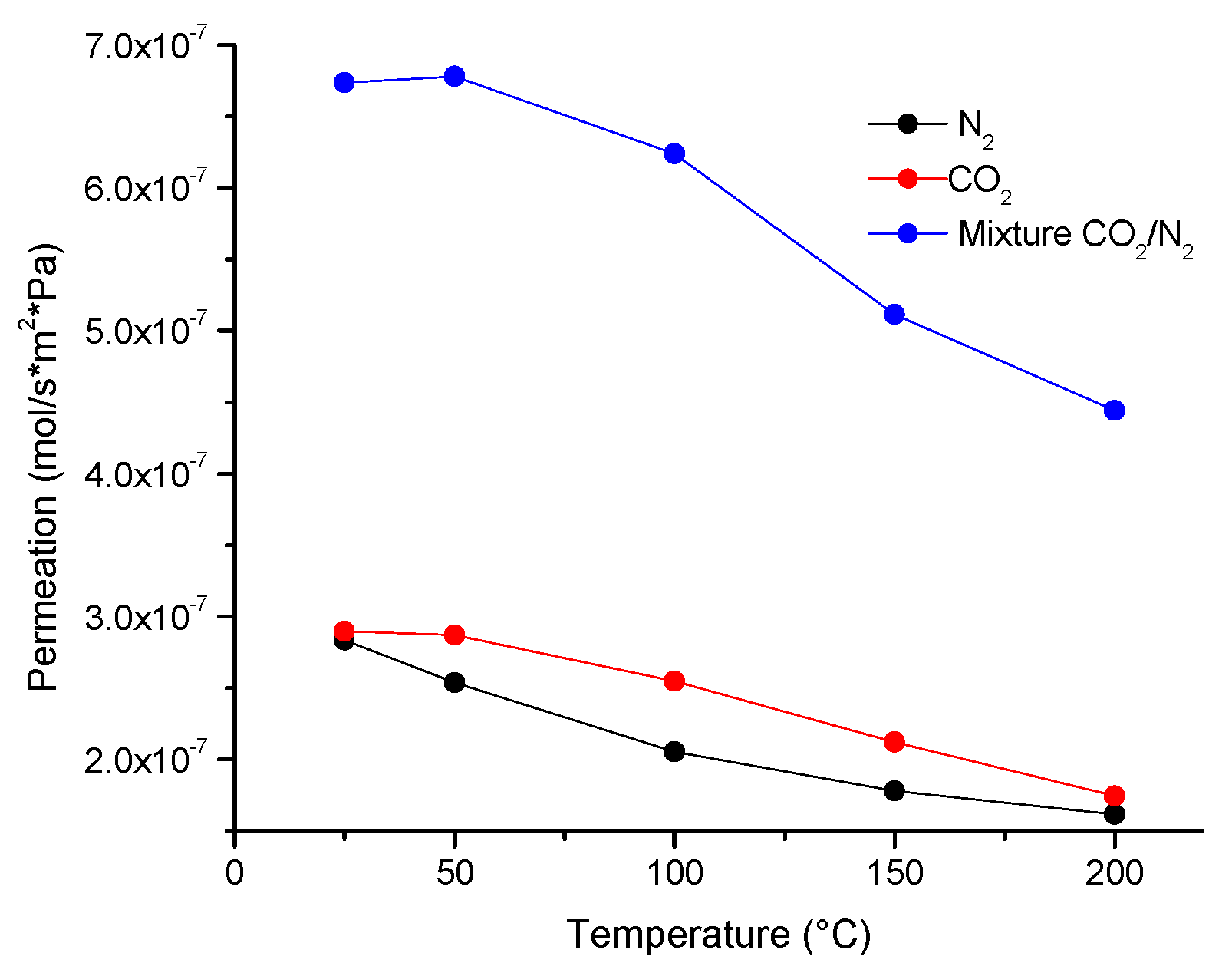
The permeation results of the simple gases N
2 and CO
2 in the membrane show a decreasing trend when temperature increases, being in the 1.6176 × 10
−7 to 2.8354 × 10
−7 mol m
−2s
−1 Pa
−1 range for N
2 and 1.7451 × 10
−7 to 2.8954 × 10
−7 mol m
−2s
−1Pa
−1 for CO
2. In the case of the CO
2/N
2 mixture, the permeation also decreases as a function of temperature; however, the permeation values are greater than that of the simple gases, namely, from 4.4404 × 10
−7 to 6.7358 × 10
−7 mol m
−2s
−1Pa
−1 (
Figure 2).
According to Bakker, et al. (1997), the temperature dependence of the flows in steady state, in the range of −83 to 407 °C through a silicalite-1 membrane, can be described using two diffusion mechanisms. Under conditions where a considerable amount of gas is adsorbed, diffusion is carried out by mass transport in an adsorbed site (surface diffusion) while at high temperatures, diffusion is described by gas transport diffusion [
8] since there is virtually no adsorption.
The results of the gases separation show a maximum separation factor (SF) of 2.1 at 25 °C, that decreased with increasing temperature, as can be seen in
Table 1.
The separation factor is the result of the composition of the permeate flow and not of the amount of flow, therefore, considering a higher molecular adsorption at low temperatures and the size of the molecules of CO2 (3.3 Å) and N2 (3.64 Å) it is presumed that the membrane performed a slightly selective separation of CO2 over the entire temperature range, due to the CO2 molecule smaller size with respect to that of N2.
3.3. Solids Recovered from Carbonation Reactions
The dry weight of the solids recovered in the carbonation reactions was recorded in order to perform a mass balance and obtain the conversion percentage of each reaction with respect to the initial concentration of the alkaline solutions and with the CO
2 flow fed. The results on conversion percentage are shown in
Table 2.
3.4. Characterization of Solids Recovered after Carbonation Reactions
The
Figure 3 shows the morphology of the solids recovered in the carbonation reactions with the different alkaline solutions as well as the elemental analysis carried out that shows the presence of carbon in the solids.
4. Conclusions
The permeation studies allowed the determination of the surface diffusion mechanism of gases through the membrane. A slight selectivity of CO2 with respect to N2 was obtained after gases separation with a factor up to 2.1 at 25 °C. Considering the mechanism of diffusion, the characteristics of the membrane, as well as the separation factor, it is concluded that the synthesis resulted in a membrane capable of separating CO2 from a CO2/N2 mixture under the study conditions, although it is possible to obtain more favorable results by chemically modifying the basic surface properties of the membrane.
The CO2 capture system shows that it is possible to obtain other compounds starting with CO2, recovering it as carbonates, and the characterization of these compounds showed that they can be used as raw material in another process.
Author Contributions
M.G.-A. and M.T.-R. conceived of and designed the experiments and analyzed the data, D.S.C.-N. performed the experiments and V.M.-Á. wrote the paper.
Acknowledgments
D. Santa Cruz Navarro is grateful for the CONACyT scholarship and the authors thank the CBI Divisional Electronic Microscopy Laboratory at UAM Azcapotzalco and the Molecular Sieve Group of the Catalysis and Petrochemistry Institute of Madrid, Spain.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Czyperek, M.; Bouwmeester, H.J.; Modigell, M.; Peinemann, K.; Voigt, I.; Meulenberg, W.A.; Singheiser, L.; Stover, D. MEM-BRAIN gas separation membranes for zero-emission fossil power plants. Energy Procedia 2009, 1, 303–310. [Google Scholar] [CrossRef][Green Version]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas. Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Yurramendi, L.; Caballero, S.; del Río, C. Los residuos industriales como reactivos para el secuestro de CO2. Afinidad: Rev. Quim. Teórica y Aplicada 2011, 68, 6–14. [Google Scholar]
- Patricio, J.; Angelis, A.; Castillo, A.; Kalmykova, Y.; Rosado, L. Region prioritization for the development of carbon capture and utilization technologies. J. CO2 Utilizat. 2017, 50–59. [Google Scholar] [CrossRef]
- Structure Commission of the International Zeolite Association, Database of Zeolite Structures. 2017. Available online: http://america.iza-structure.org/IZA-SC/pow_pat.php?STC=MFI&ID=MFI_0 (accessed on 26 June 2018).
- Bakker, W.J.; van den Broeke, L.J.; Kapteijn, F.; Moulijn, J.A. Temperature dependence of one-component permeation through a silicalite-1 membrane. AIChE J. 1997, 43, 2203–2214. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).