Active Methodologies in Chemistry †
Abstract
:1. Introduction
- -
- Provides students with a personal and professional development strategy,
- -
- helps to develop critical capacity,
- -
- favours autonomy,
- -
- engages the student in the educational process,
- -
- motivation for learning,
- -
- increases responsibility towards learning,
- -
- promotes honesty over judgements,
- -
- provides information to the teacher about their learning.
- -
- The students control their learning process,
- -
- learning can be completed at the time and place you want,
- -
- the correction and feedback task is facilitated and,
- -
- the student is not passive when interacting with the computer.
- -
- Start the student in the cooperative learning.
- -
- Motivate group work.
- -
- Motivate class attendance.
- -
- Encourage participation in the laboratory.
2. Class Methodologies
- 1Atomic structure and periodic table.
- 2Chemical bond and states of aggregation of substances.
- Stoichiometry, Solutions and Colligative properties.
- Energy of chemical reactions. Spontaneity.
- Introduction to chemical equilibrium.
- Principles of chemical kinetics.
2.1. Before the Classroom
2.2. During the Classroom
- Distribute an unfamiliar exercise to each student, a total of 6 different exercises are distributed.
- The students have 20 min to solve it individually, they can talk with the classmates they have by their side.
- For their answer they cannot ask the teacher, only clarify related to the statement of the problem.
- The teacher groups the students into four who have the same exercise. If possible, the groups must be formed by students who have not previously spoken. Two groups usually have the same exercise. There is a maximum number of twelve groups.
- For 30 min each group has to reach an agreement to resolve the same exercise
- In the last 45 min of class, the board is divided into three parts, to do three of the six exercises. Spokesperson from each group does the exercise on the board for the rest of the classmates. The spokesperson is chosen at random by the teacher. The spokesperson must solve the exercise and explain it. The score obtained by the spokesperson will be the score obtained by the group. At the same time, from the other group that has the same exercise, the teacher chooses at random another student who comes to the board and has the role of teacher. The student with the role of teacher must check if the exercise is well resolved and asks some questions to the spokesperson who has resolved the exercise before. The role of teacher is also evaluated and the score will also be the mark of the group. In summary on the blackboard we found three spokespersons solving exercises and three students with the role of teacher, it is a peer-to-peer presentation/teacher. As there are six exercises, the students chosen at random are 12, one student from each group. This activity is done in all master classes and lasts two hours. The note of this activity represents ten percent of the overall score.
2.3. After the Classroom
- The teacher distributes the exercises of the first three problem relationships among all the students in the class. Each student has to do an exercise.
- The student has a week to do it and delivers it to the e-learning platform.
- The teacher creates three blogs, one for each topic, where the solved exercises will be presented. If the exercise is well resolved, the student is allowed to upload their exercise to the blog and see the solved exercises from the other blogs or topics. If the student does not do the exercise, they will not have access to the blogs or the solved problems of the first three topics. In addition, he no longer has the option to do an exercise of the last three topics. If the student does not do the exercise well, he always has the option to receive one or several feedbacks until the resolution is correct and he can upload it to the blog.
- The exercises of the last three problem relationships are shared among all the students who have access to the blogs. One per student.
- The student has a week to do it and delivers it on the platform.
- The teacher creates the blogs of the last three topics. If the student has solved the exercise well, he will have the option to upload it to the blog, which will allow him to see all the exercises solved. If you do not have it right, you will not have an option until you solve it satisfactorily. This activity corresponds to five percent of the global mark.
3. Methodologies Followed in the Laboratory
- Law of multiple proportions. Law of conservation of mass.
- Separation of the components of a mixture.
- Preparation of solutions.
- Limiting reactant and theoretical yield.
- Chemical bond and properties of substances.
- Reaction enthalpy. Hess’s Law.
- Rate of reaction.
- Chemical equilibrium. Principle of Le Châtelier.
- Strong and weak electrolytes. Conductivity.
3.1. Before the Laboratory
3.2. During the Laboratory
- -
- Responsible for the preparation of the practice guide.
- -
- Responsible for the preparation of the practice material and solutions.
- -
- Responsible for executing the practice.
3.3. After the Laboratory
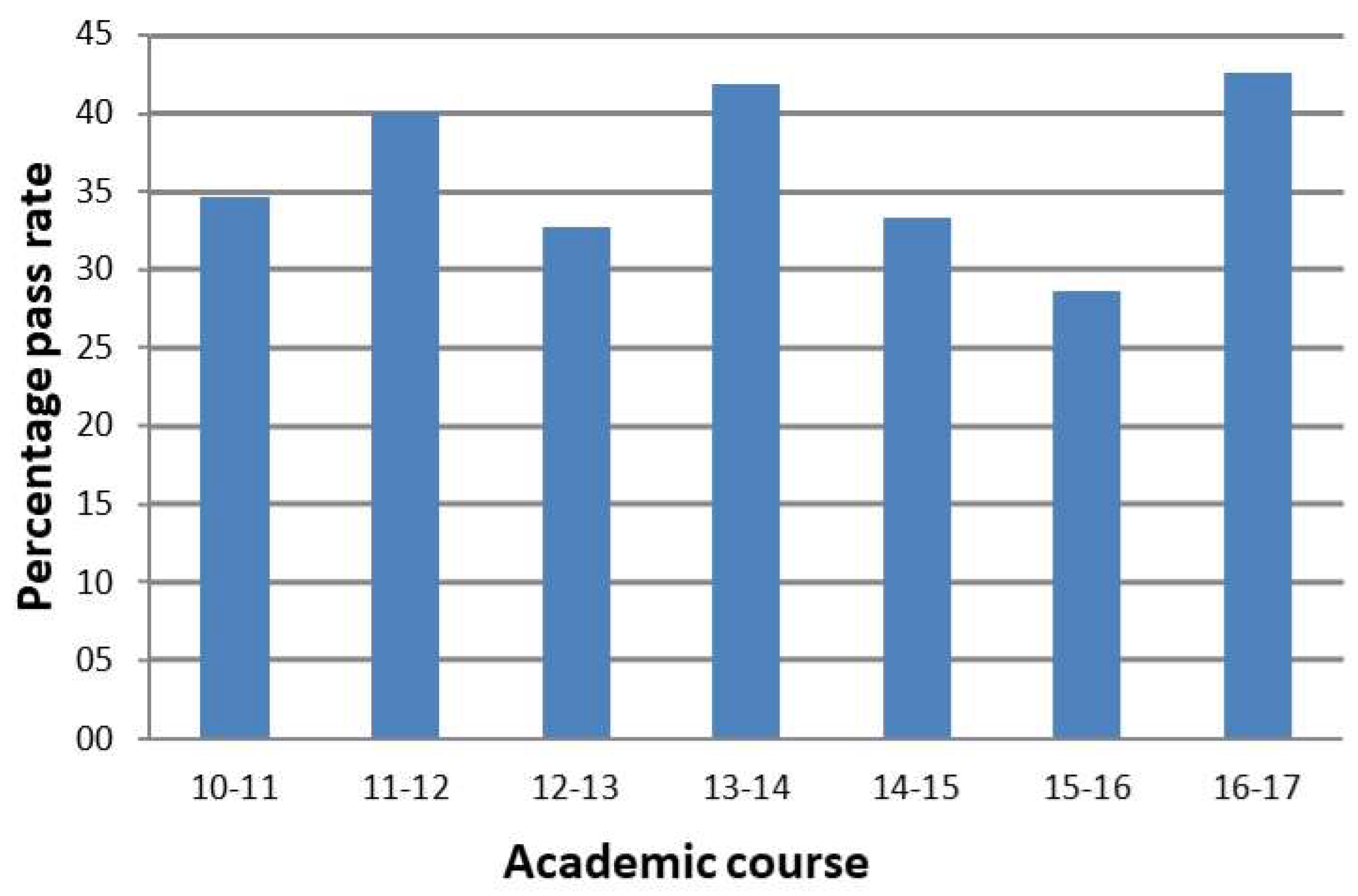
4. Results and Discussion
References
- Teo, T.W.; Tan, K.C.; Yan, Y.K.; Teo, Y.C.; Yeo, L.W. How flip teaching supports undergraduate chemistry laboratory learning. Chem. Educ. Res. Pract. 2014, 15, 550–567. [Google Scholar] [CrossRef]
- Noguera-Murray, P.; Tortajada-Genaro, L.A.; Atienza-Boronat, J.; Herrero-Villén, M.A. Auto-evaluación previa a las prácticas de laboratorio químico: Introducción al autoaprendizaje. ARBOR Ciencia Pensamiento y Cultura 2011, 187, 267–272. [Google Scholar] [CrossRef]
- Blonder, R.; Jonatan, M.; Bar-Dov, Z.; Benny, N.; Rap, S.; Sakhnini, S. Can You Tube it? Providing chemistry teachers with technological tools and enhancing their self-efficacy beliefs. Chem. Educ. Res. Pract. 2013, 14, 269–285. [Google Scholar] [CrossRef]
- Valero-Garcia, M. El método RIVeG: Resolución individual y verificación en grupo. In Primera Jornada sobre Trabajo Cooperativo; Universitat Politècnica de Catalunya, UPC: Barcelona, Spain, 2001; ISBN 84-699-8954-5. [Google Scholar]
- Ibáñez González, M.J.; Mazzuca Sobczuk, T.; Andujar Sánchez, M.; Ortiz Salmerón, E. Elaboración de Material Fotográfico en el Laboratorio de QUÍMICA I. XI Jornadas de redes de Investigación en Docencia Universitaria; Universidad de Alicante: Alicante, Spain, 2013; ISBN 978-84-695-8104-9. [Google Scholar]
| Before the Classroom: e-Learning Platform | In the Classroom | After the Classroom: e-Learning Platform |
|---|---|---|
| PowerPoint presentations, YouTube videos, audiovisual material and self-evaluations | Lectures of 20 min, resolution of exercises through informal cooperative learning | Resolution of exercises through informal cooperative learning |
| Before the Laboratory: e-Learning Platform | In the Laboratory | After the Laboratory: e-Learning Platform |
|---|---|---|
| Guide of the practices and self-evaluations | Laboratory practices and formal cooperative learning. At the end of the course, oral presentation | Portfolios of practice reports |
| Positive |
| 1. Promote the relationship between colleagues 2. Take the subject to day 3. Put in common the knowledge that each one has, helping to better understand the topic |
| Negative |
| 1. Little time 2. Failure to explain more in class 3. Go to the blackboard |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-González, M.J.; Mazzuca-Sobczuk, T. Active Methodologies in Chemistry. Proceedings 2018, 2, 1339. https://doi.org/10.3390/proceedings2211339
Ibáñez-González MJ, Mazzuca-Sobczuk T. Active Methodologies in Chemistry. Proceedings. 2018; 2(21):1339. https://doi.org/10.3390/proceedings2211339
Chicago/Turabian StyleIbáñez-González, María J., and Tania Mazzuca-Sobczuk. 2018. "Active Methodologies in Chemistry" Proceedings 2, no. 21: 1339. https://doi.org/10.3390/proceedings2211339
APA StyleIbáñez-González, M. J., & Mazzuca-Sobczuk, T. (2018). Active Methodologies in Chemistry. Proceedings, 2(21), 1339. https://doi.org/10.3390/proceedings2211339






