Abstract
In this research, we studied the chlorinated gas adsorption process using activated carbon. Two types of granular activated carbon were employed: GAC800 and GAC1200; with specific surface areas of 800 m2/g and 1200 m2/g, respectively. In order to optimize the polyvinyl chloride (PVC) dehydrochlorination conditions, three parameters were studied: (i) the type of precursor—PVC and PVC mixed with charcoal (1:1 by weight)—(ii) the temperature—300 and 400 °C—and (iii) the retention time—30, 120, and 240 min. Mohr’s method and acid-base titration were used to estimate the captured amount of chlorinated gas. The results indicated that the PVC dehydrochlorination occurred completely about 69–73 wt.% at 400 °C for 240 min. The amount of chloride ion was detected around 1–2 mmol/LNaOH. The estimated HCl adsorption capacity for the GAC800 and GAC1200 samples potentially absorbed 0.27 mgHCl/gGAC and 0.21 mgHCl/gGAC, respectively. In addition, the efficiency for GAC800 and GAC1200 was reported to 37.95% and 28.92%, respectively.
1. Introduction
Polyvinyl chloride (PVC) is one of the largest thermoplastics predominantly used all over the world. It can be combined with a number of additives to produce a wide range of end-use properties, from rigid plastics to flexible materials. In Europe, up to 30.8% of the plastic waste is landfilled, 39.5% is incinerated with the purpose of energy recovery (waste-to-energy), while only 29.7% is recycled [1,2]. The PVC waste incineration has a high impact on the environment due to the releasing of chlorinated gases. For the plastic recycling technologies, pyrolysis process (heating in an oxygen-free atmosphere) is one of the potential methods for increasing the percentage of recycled material because it allows obtaining valuable liquid fuels from plastic waste [3]. However, the release of chlorine in pyrolysis of PVC waste is of great interest. Because chlorinated compounds can be formed in the pyrolysis liquids, which is detrimental in the use of such liquids as a supply for oil refinery or to be used directly as fuels. Jordan et al. [4] reported that the dehydrochlorinated volatile compounds, called “chlorinated gas steam”, is confirmed mainly by hydrochloric gas and a small amount of benzene, toluene, along with other hydrocarbons.
In this work, a PVC stepwise-pyrolysis process was explored to optimize the reaction conditions of dehydrochlorination and to explore the effectiveness of HCl adsorption using activated carbon.
2. Sample and Experimental
2.1. Materials
Medical grade PVC round shape pellets (VM 4720 NTO) with approx. 3–4 mm in diameter was supported by the Vinlytec Industry Company (Samutprakarn, Thailand). Granular activated carbon (GAC) derived from coconut shells used in this study was provided by Carbokarn Co., Ltd. (Bangkok, Thailand). The size of GAC absorbent was about 2.36–4.75 mm in diameter with two specific surface areas of 800 m2/g (GAC800) and 1200 m2/g (GAC1200).
2.2. PVC Dehydrochlorination and Analysis
PVC dehydrochlorination process was performed using a fixed-bed reactor under atmospheric pressure at 300 °C and 400 °C. The loading amount of PVC compound for each run was 2.5 g. To study the effect of heating media, charcoal powder was added in the ratio of 1:1 by weight. Pure N2 gas (commercial grade) was introduced at a rate of 15 mL/min for 15 min. The experiment was done for both with 25 g GAC solid absorbent and without GAC (called “blank experimentation”) for 30, 120, and 240 min. An amount of 250 mL of 0.75 M NaOH solution was used to capture the rest chlorinated gas. The collected NaOH solution was further analyzed for the chloride ion absorbed amount (Mohr’s method using AgNO3 solution) and for remained NaOH concentration (acid-base titration).
To estimate the activated carbon adsorption capacity and efficiency, we use the following equations:
where and are the chloride ion amount (mole) in the NaOH solution when running without and with GAC absorbent, respectively.
3. Result and Discussion
3.1. Percentage of PVC Weight Decomposition
Figure 1a shows TGA curve of PVC compound under N2 atmosphere. There are two stages for the PVC decomposition: the first stage occurs between 235 °C and 365 °C with a weight loss of 65 wt.%. This initial decomposition is due to the dehydrochlorination reaction of the polymer forming de-HCl PVC and volatile compounds [2,4,5]—the latter mainly consists of HCl and a few hydrocarbons, as shown in Equation (3). In the second stage, the extending decomposition from 365 to 525 °C shows a weight loss of 20%. It corresponds to the cracking and decomposition of de-HCl PVC [2].
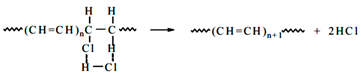
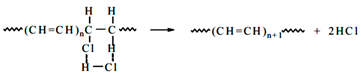
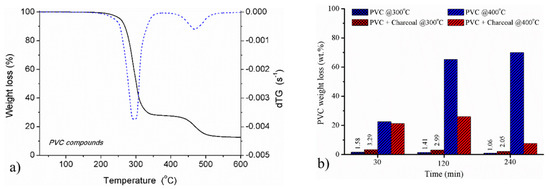
Figure 1.
(a) TGA curve (solid line) and first derivative of the TGA curve (dashed line) of PVC compound, (b) PVC weight-loss percentage with/without heating-media (charcoal) using stepwise-pyrolysis at 300 °C and 400 °C.
A minimum temperature for dehydrochlorination of PVC revealed about 300 °C, see in TGA curve. However, the stepwise-pyrolysis reactor used in this research has a larger volume than the TGA apparatus, which might reduce the completion of dehydrochlorination reaction. The PVC dehydrochlorination performed with the stepwise-pyrolysis apparatus was hereby done at two temperatures: 300 °C and 400 °C. Figure 1b illustrates the weight loss percentage of PVC dehydrochlorination at 300 °C and 400 °C with/without charcoal heating media. The maximum PVC weight loss of 65% was carried out without charcoal at 400 °C for 240 min., the result is comparable to the result obtained with TGA analysis. However, adding charcoal gave a lower percentage of PVC weight loss (25 wt.%), about three-time less than without charcoal, indicating that this addition does not improve the dehydrochlorination reaction.
3.2. Chloride-Ion Quantitative Analysis
The concentration of chloride ion [Cl−] absorbed in 1 L NaOH was analyzed as shown in Figure 2a. The concentration of chloride ion was increased as a function of dehydrochlorination time. However, the maximum of 2 mmol of Cl− was observed correspond to the completion of the PVC dehydrochlorination (65 wt.% lost). With the max. released [Cl−], the amount of NaOH approximate 64 mmol was used. It is in a contrast to the basic reaction of HCl vs. NaOH—1 mole HCl reacts with 1 mole NaOH. According to Jordan et al. [4], the remaining compounds being absorbed in NaOH solution might be some other light hydrocarbons.
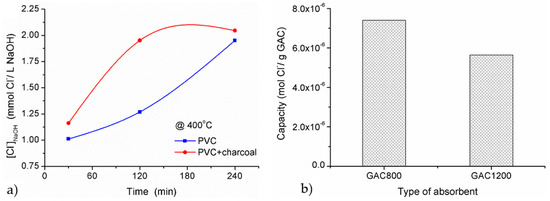
Figure 2.
Quantitative analysis: (a) the amount of chloride ion captured in NaOH solution (blue) and without (red) charcoal heating media at 400 °C, (b) capacity of chlorinated gas adsorption.
3.3. Chlorinated Gas Adsorption Capacity
The adsorption capacity for GAC800 and GAC1200 absorbent were estimated in ca. of dehydrochlrination at 400 °C for 240 min. As it can be seen in Figure 2b, the adsorption capacity for both GAC800 absorbent revealed higher than that of GAC1200. Based on the amount of HCl moiety, the adsorption capacity of the GAC800 and GAC1200 sample allowed absorbing approximate 5.4 × 10−4 gHCl/gGAC and 4.1 × 10−4 gHCl/gGAC respectively. In fact, the granular activated carbon with the lower specific surface area is more appropriate for a gas-sorption treatment. The efficiency of GAC800 and GAC1200 were further calculated using Equation (2). It reported approximate 37.95% and 28.92% for GAC800 and GAC1200, respectively.
4. Conclusions
The dehydrochlorination of PVC compound was possible under N2 atmosphere using a fixed-bed reactor. The optimized temperature and time for the dehydrochlorination of 2.5 g of PVC were 400 °C and 240 min. The assisted dehydrochlorination using charcoal as heating media gave an insignificant contribution to the completion of the reaction. According to the optimized conditions, GAC with a specific surface area of 800 m2/g and 1200 m2/g absorbed approximate 0.54 mgHCl/gGAC and 0.41 mgHCl/gGAC, respectively. The GAC800 absorbent was more suitable for the PVC dehydrochlorination process.
Acknowledgments
The author would like to acknowledge Hataichanok Krimongkol and Kitiwan Sae-Jiw for helping on experimentation. The Research and Development (Grant No. WJP. 4/2560) and the fiscal year’s project for the undergraduate program in chemical engineering (Engineering faculty) are gratefully acknowledged for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sadat-Shojai, M.; Bakhshandeh, G.R. Sadat-Shojai. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Dechlorination of fuels in pyrolysis of PVC containing plastic wastes. Fuel Process. Technol. 2011, 92, 253–260. [Google Scholar] [CrossRef]
- Jordan, K.J.; Suib, S.L.; Koberstein, J.T. Determination of the degradation mechanism from the kinetic parameters of dehydrochlorinated poly(vinyl chloride) decomposition. J. Phys. Chem. B 2001, 105, 3174–3181. [Google Scholar] [CrossRef]
- Troitskii, B.B.; Troitskaya, L.S. Degenerated branching of chain in poly(vinyl chloride) thermal degradation. Eur. Polym. J. 1999, 35, 2215–2224. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).