Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oleic Acid Coating
2.2. Silica Dioxide Coating
3. Result and Discussion
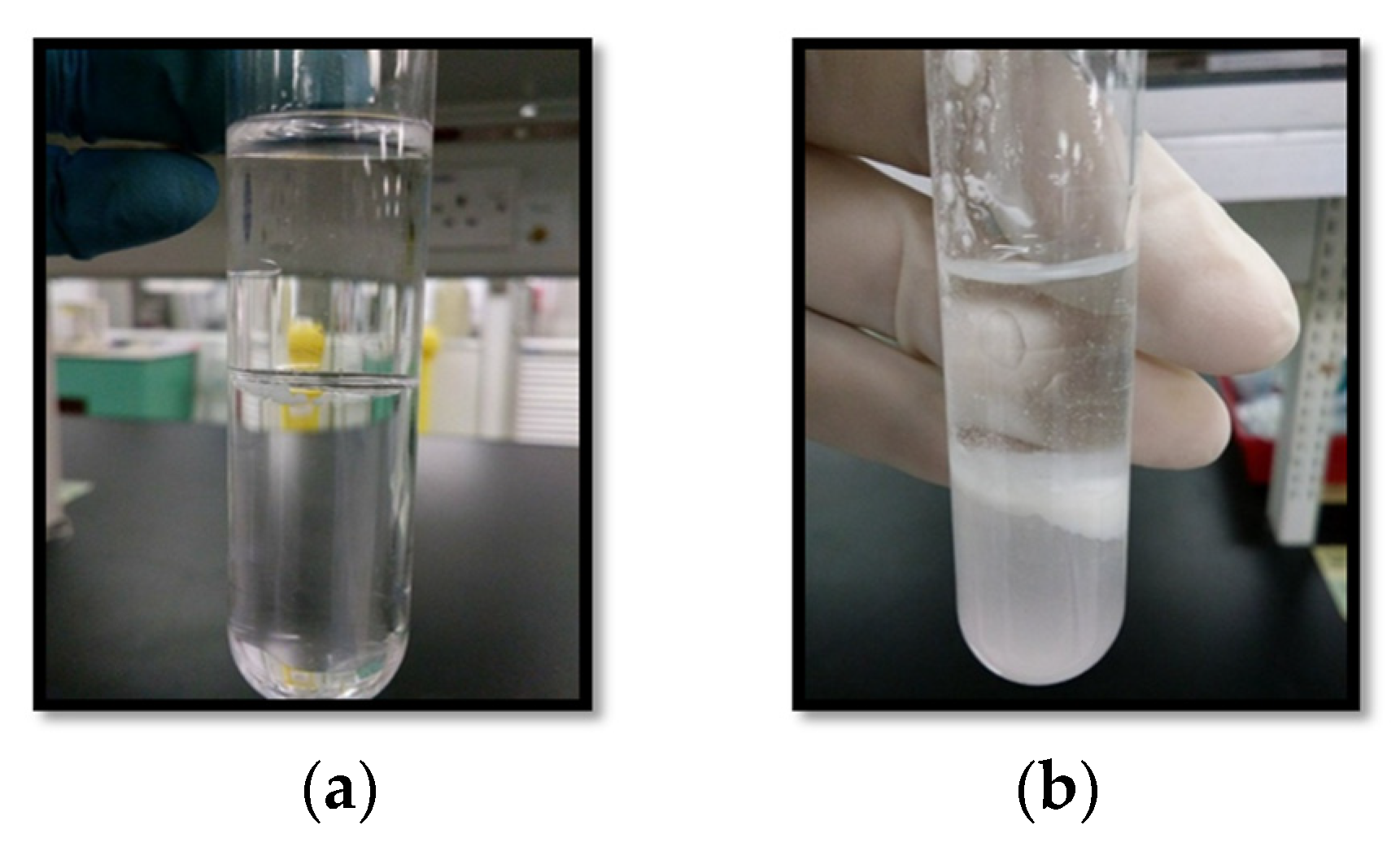
3.1. Observation Analysis
3.2. SEM, FTIR and XRD Analysis
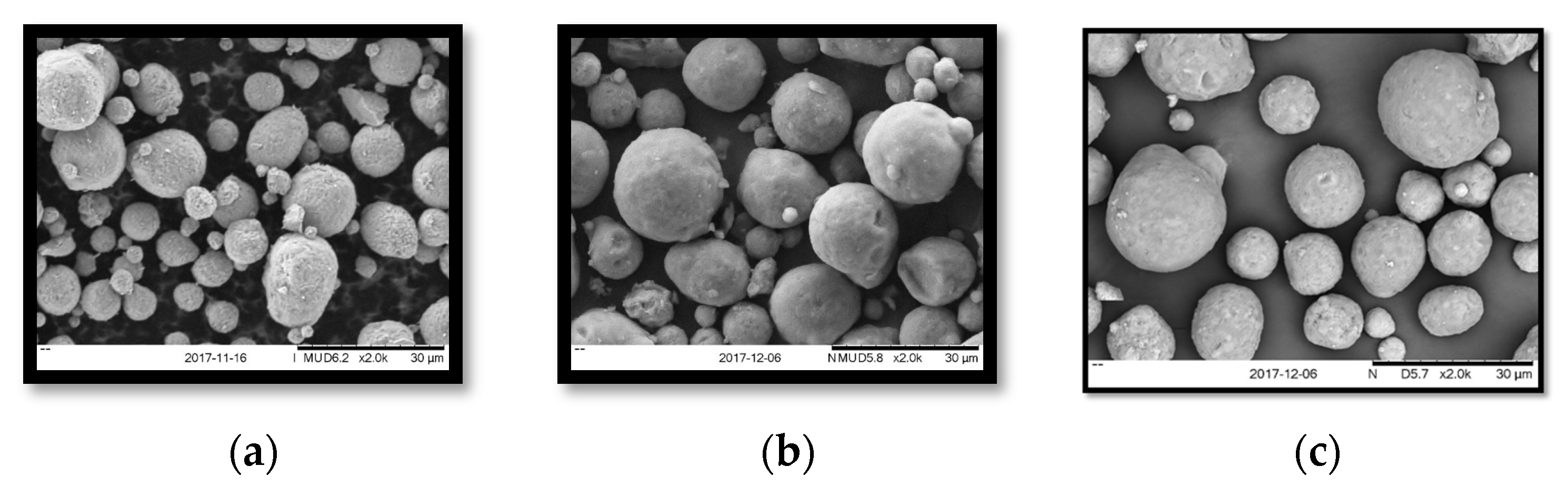
3.2.1. SEM Images
3.2.2. FTIR Analysis
3.2.3. XRD Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dalla Pria, P. Evolution and new application of the alumina ceramics in joint replacement. Eur. J. Orthop. Surg. Traumatol. 2007, 17, 253–256. [Google Scholar] [CrossRef]
- Dillon, A.C.; Mahan, A.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; Lee, S.H. Metal Oxide nano-particles for improved electrochromic and lithium-ion battery technologies. Thin Solid Films 2008, 516, 794–797. [Google Scholar] [CrossRef]
- Piriyawong, V.; Thongpool, V.; Asanithi, P.; Limsuwan, P. Preparation and Characterization of Alumina Nanoparticles in Deionized Water Using Laser Ablation Technique. J. Nanomater. 2012, 2012, 2. [Google Scholar] [CrossRef]
- Ferris, R. Nanotechnology in the Oil & Gas Industry. Retrieved from Mastinc. Available online: http://mastinc.com/nanotechnology-in-the-oil-gas-industry/ (accessed on 2 December 2017).
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Philos. Trans. R. Soc. A 2014, 372, 20120320. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zeng, F.; Chang, X.; Liu, H. A systematic integrated approach for waterflooding optimization. J. Pet. Sci. Eng. 2013, 112, 129–138. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Maryam Lashanizadegan, G.F. Synthesis and surface modification of aluminium oxide nanoparticles. J. Ceram. Process. Res. 2014, 15, 316–319. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm–1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Speakman, S.A. Introduction to X-Ray Powder Diffraction Data Analysis; Massachusettes Institute of Technology: Cambridge, MA, USA, 2013. [Google Scholar]
- Cullity, B.D. Elements of X Ray Diffraction; Addison-Wesley Publishing Company, Inc.: Boston, MA, USA, 1956. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, M.Z.M.; Sollahunddin, N.A.; Irawan, S. Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production. Proceedings 2018, 2, 1273. https://doi.org/10.3390/proceedings2201273
Noor MZM, Sollahunddin NA, Irawan S. Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production. Proceedings. 2018; 2(20):1273. https://doi.org/10.3390/proceedings2201273
Chicago/Turabian StyleNoor, Mohd Zulkifli Mohamad, Najaatul Aimi Sollahunddin, and Sonny Irawan. 2018. "Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production" Proceedings 2, no. 20: 1273. https://doi.org/10.3390/proceedings2201273
APA StyleNoor, M. Z. M., Sollahunddin, N. A., & Irawan, S. (2018). Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production. Proceedings, 2(20), 1273. https://doi.org/10.3390/proceedings2201273





