Associations between Commonly Used Characteristics in Frailty Assessment and Mental State in Frail Elderly People †
Abstract
:1. Introduction
2. Objective
3. Materials and Methods
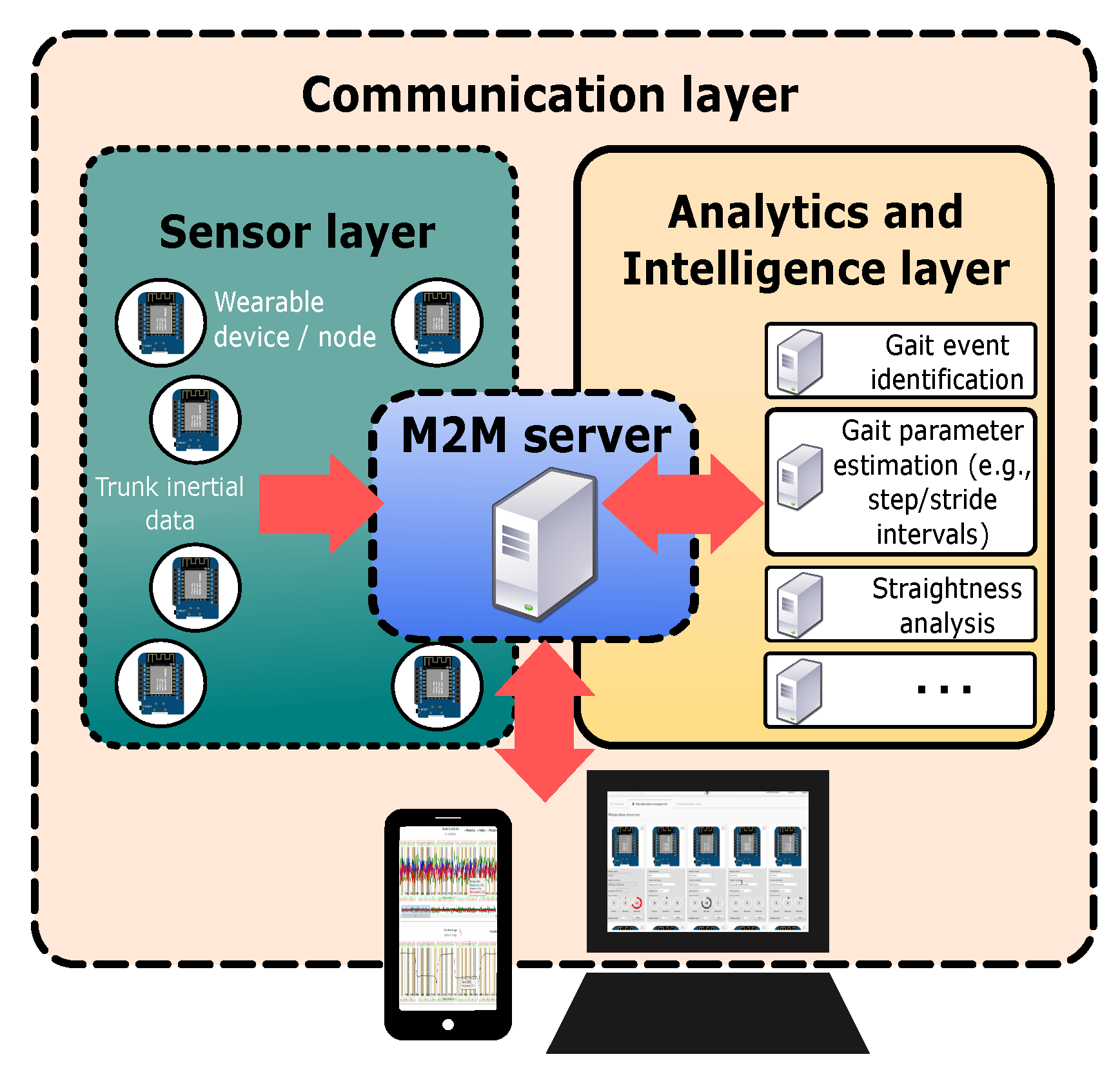
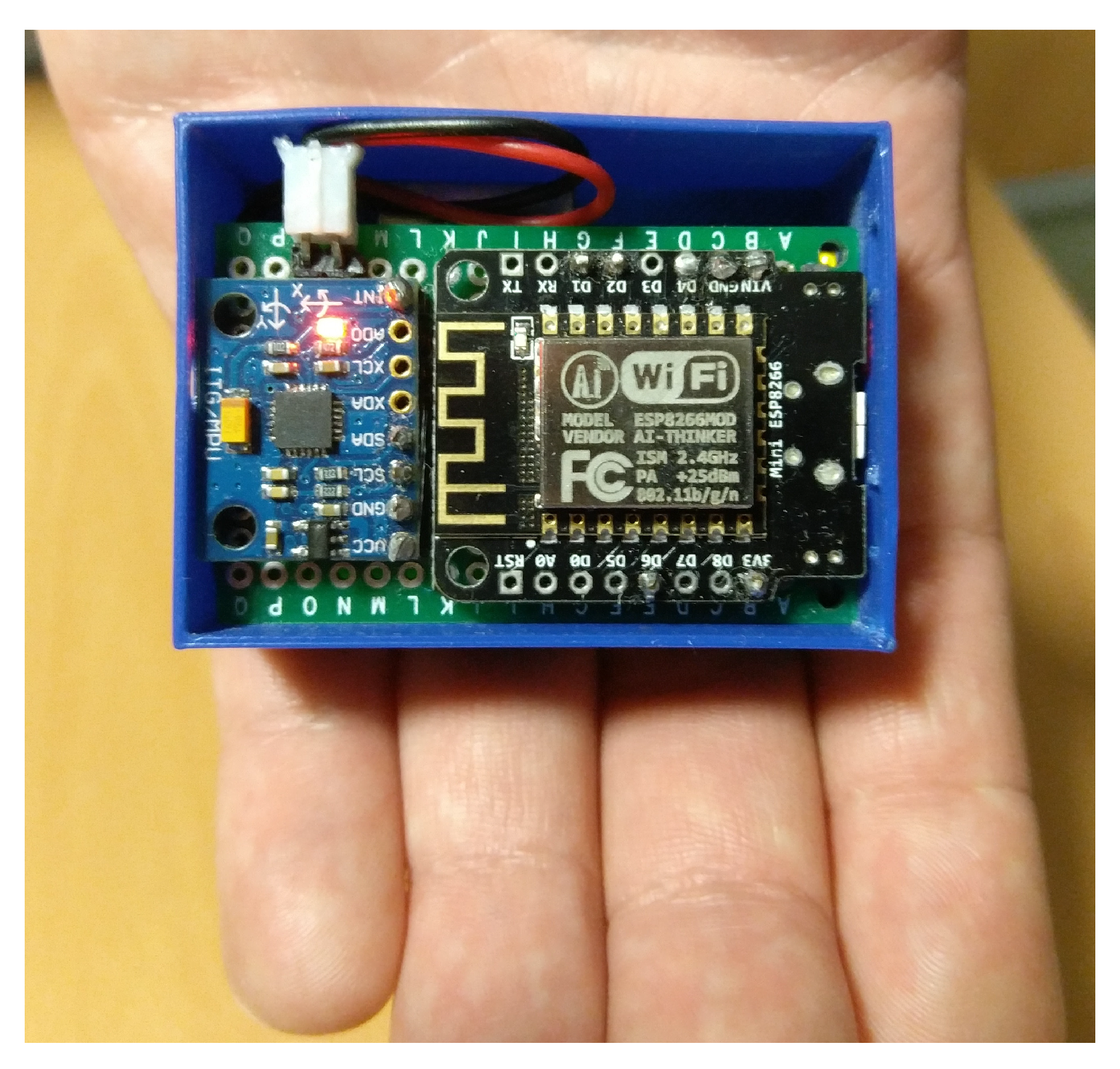
3.1. Instrumentation
3.2. Subjects, Variables and Protocol
3.3. Descriptive Statistical Analysis
3.4. Multiple Logistic Regression
4. Results
5. Discussion
6. Conclusions and Future Work
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rizos, L.R.; Soler, P.A. Fragilidad como predictor de episodios adversos en estudios epidemiológicos: RevisióN de la literatura. Revista Española de Geriatría y Gerontología 2013, 48, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Avila-Funes, J.; Helmer, C.; Amieva, H.; Barberger-Gateau, P.; Goff, M.L.; Raoux, N.; Ritchie, K.; Carrière, I.; Tavernier, B.; Tzourio, C.; et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: The three-city study. J. Am. Geriatr. Soc. 2009, 57, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2412. [Google Scholar] [CrossRef]
- Samper-Ternent, R.; Snih, S.A.; Raji, M.A.; Markides, K.S.; Ottenbacher, K.J. Relationship between frailty and cognitive decline in older Mexican Americans. J. Am. Geriatr. Soc. 2008, 56, 1845–1852. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef]
- Chong, M.S.; Tay, L.; Chan, M.; Lim, W.S.; Ye, R.; Tan, E.K.; Ding, Y.Y. Prospective longitudinal study of frailty transitions in a community-dwelling cohort of older adults with cognitive impairment. BMC Geriat. 2015, 15. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Chon, D.; Lee, K.E.; Kim, J.H.; Myeong, S.; Kim, S. The effects of frailty and cognitive impairment on 3-year mortality in older adults. Maturitas 2018, 107, 50–55. [Google Scholar] [CrossRef]
- Feng, L.; Nyunt, M.S.Z.; Gao, Q.; Feng, L.; Yap, K.B.; Ng, T.P. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies (SLAS). JAMDA 2017, 18, 252–258. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lobo, A. El Mini Examen Cognoscitivo, un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes psiquiátricos. J. Rev. Psiquiatr. Psicol. Med. 1980, 14, 39–57. [Google Scholar]
- Hilbe, J.M. Logistic Regression. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 755–758. [Google Scholar] [CrossRef]
- González, I.; Fontecha, J.; Bravo, J. Relationship between stride interval variability and aging: Use of linear and non-linear estimators for gait variability assessment in assisted living environments. JAIHC 2017, 1–15. [Google Scholar] [CrossRef]
- González, I.; Fontecha, J.; Hervás, R.; Bravo, J. Estimation of Temporal Gait Events from a Single Accelerometer Through the Scale-Space Filtering Idea. J. Med. Syst. 2016, 40, 251. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarade, J. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Casanovas, A.S. The Mini Nutritional Assessment. Twenty years contributing to nutritional assessment. Rev. Esp. Geriatr. Gerontol. 2012, 47, 245–246. [Google Scholar] [CrossRef]
- Mahoney, F.; Barthel, D. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 56–61. [Google Scholar]
- Collin, C.; Wade, D.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Cholesterol Levels: What You Need to Know. In NIH Medline Plus; 2012. Available online: https://medlineplus.gov/magazine/issues/summer12/articles/summer12pg6-7.html (accessed on 16 October 2018).
- Muir, S.W.; Speechley, M.; Wells, J.; Borrie, M.; Gopaul, K.; Montero-Odasso, M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture 2012, 35, 96–100. [Google Scholar] [CrossRef]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2016, 63, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Launay, C.P.; Sekhon, H.; Barthelemy, J.C.; Roche, F.; Chabot, J.; Levinoff, E.J.; Allali, G. Association of increased gait variability while dual tasking and cognitive decline: Results from a prospective longitudinal cohort pilot study. GeroScience 2017, 39, 439–445. [Google Scholar] [CrossRef] [PubMed]
- González, I.; López-Nava, I.H.; Fontecha, J.; Muñoz-Meléndez, A.; Pérez-SanPablo, A.I.; Quiñones-Urióstegui, I. Comparison between passive vision-based system and a wearable inertial-based system for estimating temporal gait parameters related to the GAITRite electronic walkway. J. Biomed. Inform. 2016, 62, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ikeda, S.; Nakahara, M.; Ikeda, T.; Okamoto, R.; Kurosawa, K.; Horikawa, E. Factors affecting the coefficient of variation of stride time of the elderly without falling history: A prospective study. JPTS 2015, 27, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Owings, T.M.; Grabiner, M.D. Variability of step kinematics in young and older adults. Gait Posture 2004, 20, 26–29. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 October 2018).
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1995, 82, 667–667. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef]
| Variable | Category | n(%) |
|---|---|---|
| Age (in years) | ||
| Sex | female | |
| male | ||
| BMI(%) a | normal | |
| overweight | ||
| obese | ||
| MNA(/30) b | normal | |
| risk | ||
| malnutrition | ||
| Leukocytes (L) | low | |
| normal | ||
| high | ||
| Lymphocytes (L) | low | |
| normal | ||
| high | ||
| Proteins (g/dL) | low | |
| normal | ||
| high | ||
| Cholesterol (mg/dL) | normal | |
| high | ||
| very high | ||
| Barthel scale (/100) | moderate dependence | |
| mild dependence | ||
| independent 100 | ||
| Tinetti test (/28) | absence risk of fall | |
| moderate risk | ||
| severe risk | ||
| Stride interval mean | ||
| (msec) | ||
| Stride interval CV(%) c | ||
| MMSE(/35) d | impairement | |
| no impairement |
| MMSE (Mental State) | |||||
|---|---|---|---|---|---|
| Variable | Category | n(%) | NO Cognit. Impairment (%) | Cognit. Impairment (%) | p-Value a |
| Age (in years) | (*) | ||||
| Sex | female | ||||
| male | |||||
| BMI(%) b | normal | ||||
| overweight | |||||
| obese | |||||
| MNA(/30) c | normal | ||||
| risk | |||||
| Leukocytes (L) | low | ||||
| normal (4500, 11,000) | |||||
| high | |||||
| Lymphocytes (L) | low | ||||
| normal | |||||
| high | |||||
| Proteins (g/dL) | low | ||||
| normal | |||||
| Cholesterol (mg/dL) | normal | ||||
| high | |||||
| Barthel scale (/100) | moderate depend. | (*) | |||
| mild dependence | |||||
| independent 100 | |||||
| Variable | Category | n(%) | NO Cognit. Impairment(%) | Cognit. Impairment(%) | p-Value a |
| Tinetti test(/28) | absence risk of fall | (*) | |||
| moderate risk | |||||
| severe risk | |||||
| Stride interval mean | |||||
| (msec) | |||||
| Stride interval CV(%) d | (*) | ||||
| Variable | Category | p-value | OR a | 95% CI b |
|---|---|---|---|---|
| Age (in years) | (ref) | - | 1 | − |
| 0.36 | ||||
| 1.11 | ||||
| Barthel scale(/100) | independent(ref) | - | 1 | − |
| mild dependence | ||||
| moderate dependence | ||||
| Tinetti test(/28) | absence risk of fall(ref) | - | 1 | − |
| moderate risk | ||||
| severe risk | ||||
| Stride interval CV(%) | (ref) | - | 1 | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, I.; Garrido, R.D.l.C.; Navarro, F.J.; Fontecha, J.; Hervás, R.; Bravo, J. Associations between Commonly Used Characteristics in Frailty Assessment and Mental State in Frail Elderly People. Proceedings 2018, 2, 1247. https://doi.org/10.3390/proceedings2191247
González I, Garrido RDlC, Navarro FJ, Fontecha J, Hervás R, Bravo J. Associations between Commonly Used Characteristics in Frailty Assessment and Mental State in Frail Elderly People. Proceedings. 2018; 2(19):1247. https://doi.org/10.3390/proceedings2191247
Chicago/Turabian StyleGonzález, Iván, Rocío De la Cruz Garrido, Fco Javier Navarro, Jesús Fontecha, Ramón Hervás, and José Bravo. 2018. "Associations between Commonly Used Characteristics in Frailty Assessment and Mental State in Frail Elderly People" Proceedings 2, no. 19: 1247. https://doi.org/10.3390/proceedings2191247
APA StyleGonzález, I., Garrido, R. D. l. C., Navarro, F. J., Fontecha, J., Hervás, R., & Bravo, J. (2018). Associations between Commonly Used Characteristics in Frailty Assessment and Mental State in Frail Elderly People. Proceedings, 2(19), 1247. https://doi.org/10.3390/proceedings2191247







