Solvent Resistant O2 Sensor Integrated in Pressured Flow Reactors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Preparation and Integration
2.2. Sensor Response
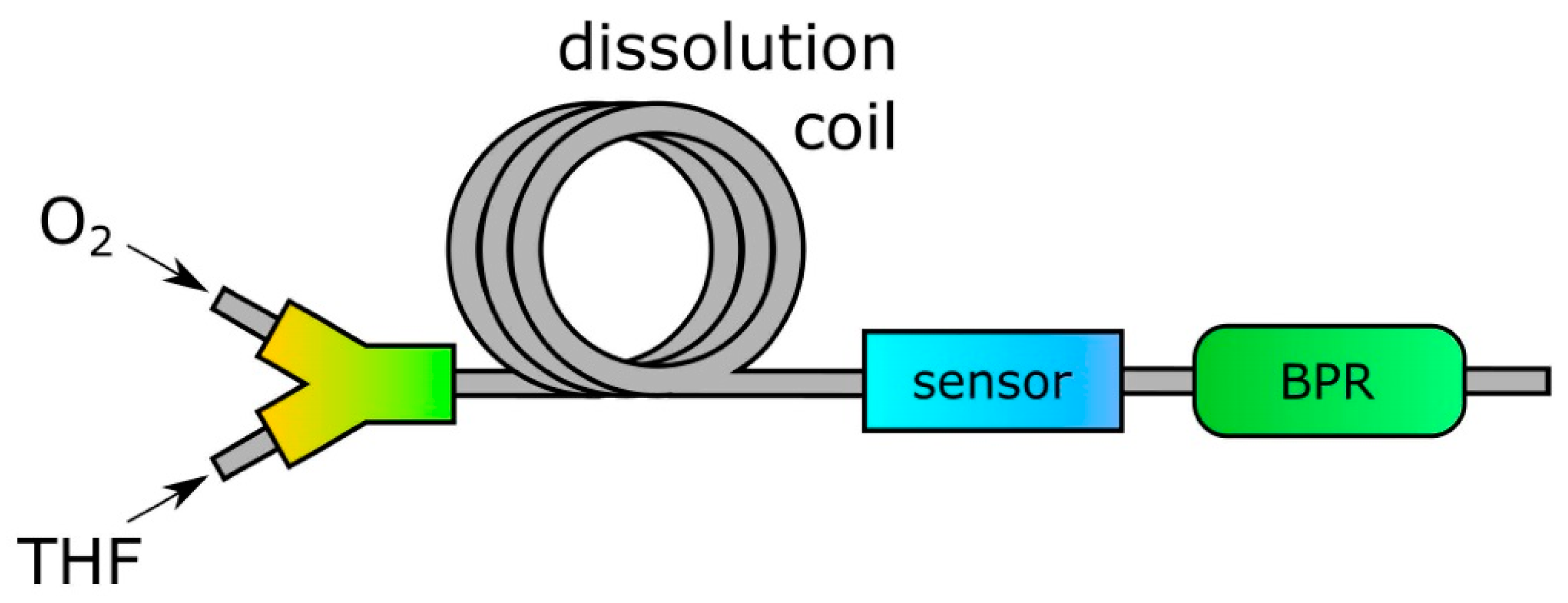
2.3. Monitoring of Oxygen Consumption
3. Results
3.1. Sensor Calibration
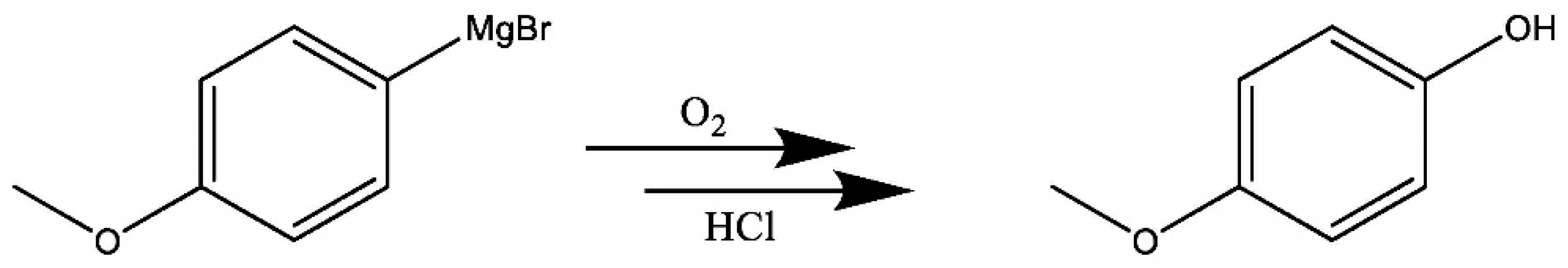
3.2. Reaction Monitoring
4. Discussion
Acknowledgments
References
- Chu, C.; Lo, Y.; Sung, T. Review on Recent Developments of Fluorescent Oxygen and Carbon Dioxide Optical Fiber Sensors. Photonic Sens. 2011, 1, 234–250. [Google Scholar] [CrossRef]
- Pedersen, A.T.; de Carvalho, T.M.; Sutherland, E.; Rehn, G.; Ashe, R.; Woodley, J.M. Biotechnol. Bioeng. 2017, 114, 1222–1230. [CrossRef] [PubMed]
- Koren, K.; Borisov, S.M.; Klimant, I. Stable optical oxygen sensing materials based on click-coupling of fluorinated platinum(II) and palladium(II) porphyrins—A convenient way to eliminate dye migration and leaching. Sens. Actuators B Chem. 2012, 169, 173–181. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulzer, P.; Lebl, R.; Mayr, T. Solvent Resistant O2 Sensor Integrated in Pressured Flow Reactors. Proceedings 2018, 2, 994. https://doi.org/10.3390/proceedings2130994
Sulzer P, Lebl R, Mayr T. Solvent Resistant O2 Sensor Integrated in Pressured Flow Reactors. Proceedings. 2018; 2(13):994. https://doi.org/10.3390/proceedings2130994
Chicago/Turabian StyleSulzer, Philipp, René Lebl, and Torsten Mayr. 2018. "Solvent Resistant O2 Sensor Integrated in Pressured Flow Reactors" Proceedings 2, no. 13: 994. https://doi.org/10.3390/proceedings2130994
APA StyleSulzer, P., Lebl, R., & Mayr, T. (2018). Solvent Resistant O2 Sensor Integrated in Pressured Flow Reactors. Proceedings, 2(13), 994. https://doi.org/10.3390/proceedings2130994




