Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate †
Abstract
:1. Introduction
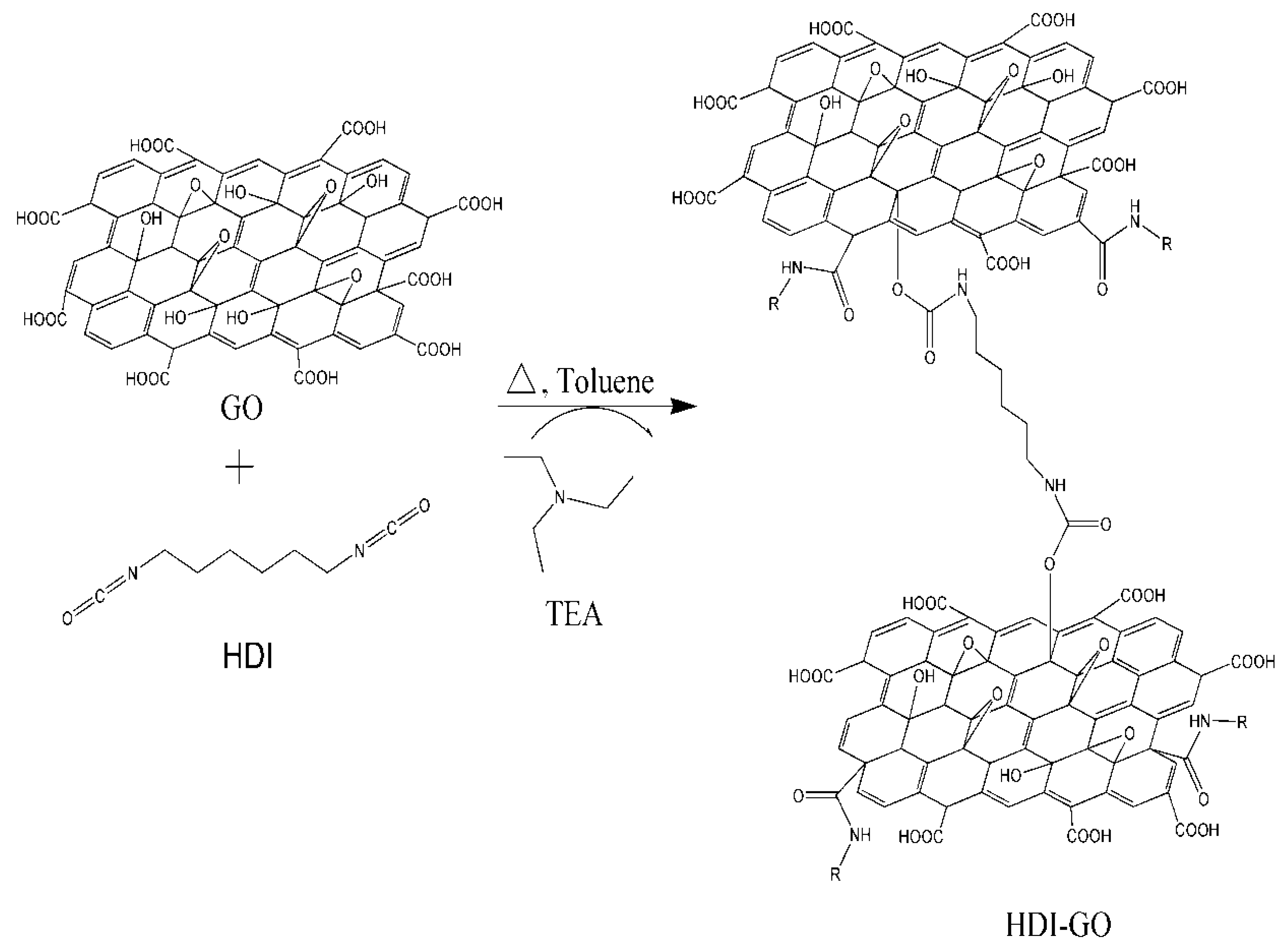
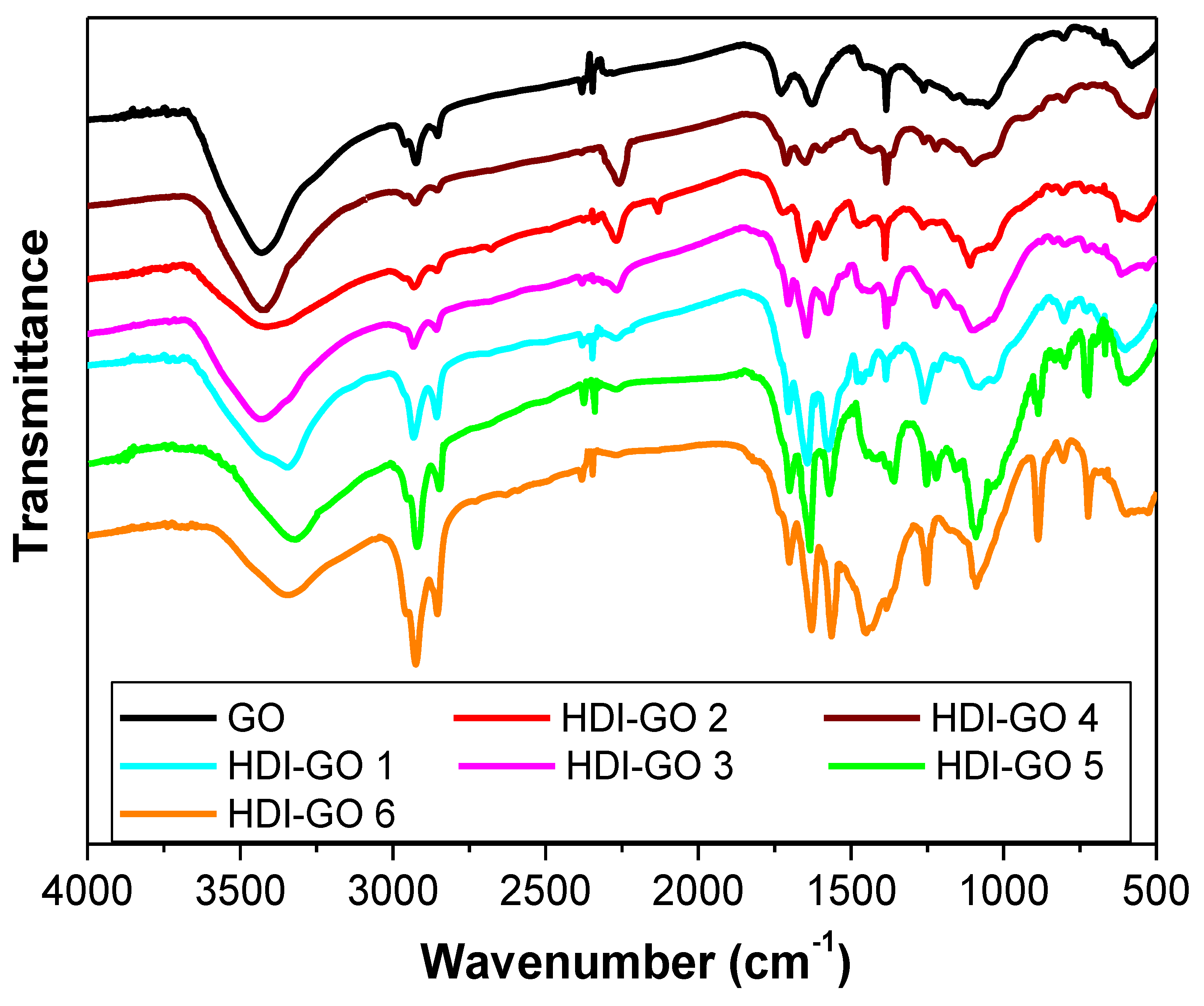
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Graphene Oxide (GO)
3.3. Synthesis of Hexamethylene Diisocyanate-Functionalized Graphene Oxide (HDI-GO)
3.4. Instrumentation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Poly(propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 7902–17914. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in Polymer Nanocomposites. Prog. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef]
- Salavagione, H.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gomez-Fatou, M. Chemical sensors based on polymer composites with carbon nanotubes and G. The role of the polymer. J. Mater. Chem. 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. , Luceño Sánchez, J.A., Peña Capilla, R., García Díaz, P. Recent advances in graphene/polymer nanocomposites for applications in polymer solar cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Aliotkhazraei, M.; Ali, N.; Milne, W.I.; Ozkan, C.S.; Mitura, S.; Gervasoni, J.L. Graphene Science Handbook: Fabrication Methods; CRC Press: Boca Raton, UK, 2016; ISBN 9781466591271. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Titelman, G.I.; Gelman, V.; Bron, S.; Khalfin, R.L.; Cohen, Y.; Bianco-Peled, H. Characteristics and microstructure of aqueous colloidal dispersions of graphite oxide. Carbon 2005, 43, 641–649. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Rouff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Dekany, I. DRIFT study of deuterium-exchanged graphite oxide. Carbon 2005, 43, 3186–3189. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.; Fateley, W.; Grasselli, J. The Handbook of Infrared and Raman Characteristic Frequencies of Organic molecules; Academic Press: San Diego, United States, 1991; ISBN 9780080571164. [Google Scholar]
- Cannon, C.G. Infrared frequencies of amide, urea, and urethane groups. J. Phys. Chem. 1976, 80, 1247–1248. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; García-García, D.; San Andrés, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
| Sample | Reaction Time (h) | Reaction Temperature (°C) | GO/HDI/Triethylamine (TEA) Weight Ratio | Tip/Bath Sonication Time (min) | Solvent Volume (mL) |
|---|---|---|---|---|---|
| GO | _ | _ | _ | _ | _ |
| HDI-GO 1 | 12 | 60 | 1/1/1 | 0/120 | 25 |
| HDI-GO 2 | 12 | 60 | 0.5/1/1 | 0/120 | 25 |
| HDI-GO 3 | 48 | 60 | 1/1/1 | 0/120 | 25 |
| HDI-GO 4 | 12 | 90 | 1/1/1 | 0/120 | 25 |
| HDI-GO 5 | 12 | 60 | 1/1/1 | 5/120 | 50 |
| HDI-GO 6 | 12 | 60 | 1/1/1 | 5+5+5*/120 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luceño-Sánchez, J.A.; Maties, G.; Gonzalez-Arellano, C.; Díez-Pascual, A.M. Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate. Proceedings 2019, 3, 8. https://doi.org/10.3390/IOCN_2018-1-05485
Luceño-Sánchez JA, Maties G, Gonzalez-Arellano C, Díez-Pascual AM. Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate. Proceedings. 2019; 3(1):8. https://doi.org/10.3390/IOCN_2018-1-05485
Chicago/Turabian StyleLuceño-Sánchez, José A., Georgiana Maties, Camino Gonzalez-Arellano, and Ana M. Díez-Pascual. 2019. "Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate" Proceedings 3, no. 1: 8. https://doi.org/10.3390/IOCN_2018-1-05485
APA StyleLuceño-Sánchez, J. A., Maties, G., Gonzalez-Arellano, C., & Díez-Pascual, A. M. (2019). Synthesis and Characterization of Graphene Oxide Derivatives via Functionalization Reaction with Hexamethylene Diisocyanate. Proceedings, 3(1), 8. https://doi.org/10.3390/IOCN_2018-1-05485






