Fabrication of Structured Boron-Doped Diamond Films for Electrochemical Applications †
Abstract
:1. Introduction
2. Materials and Methods
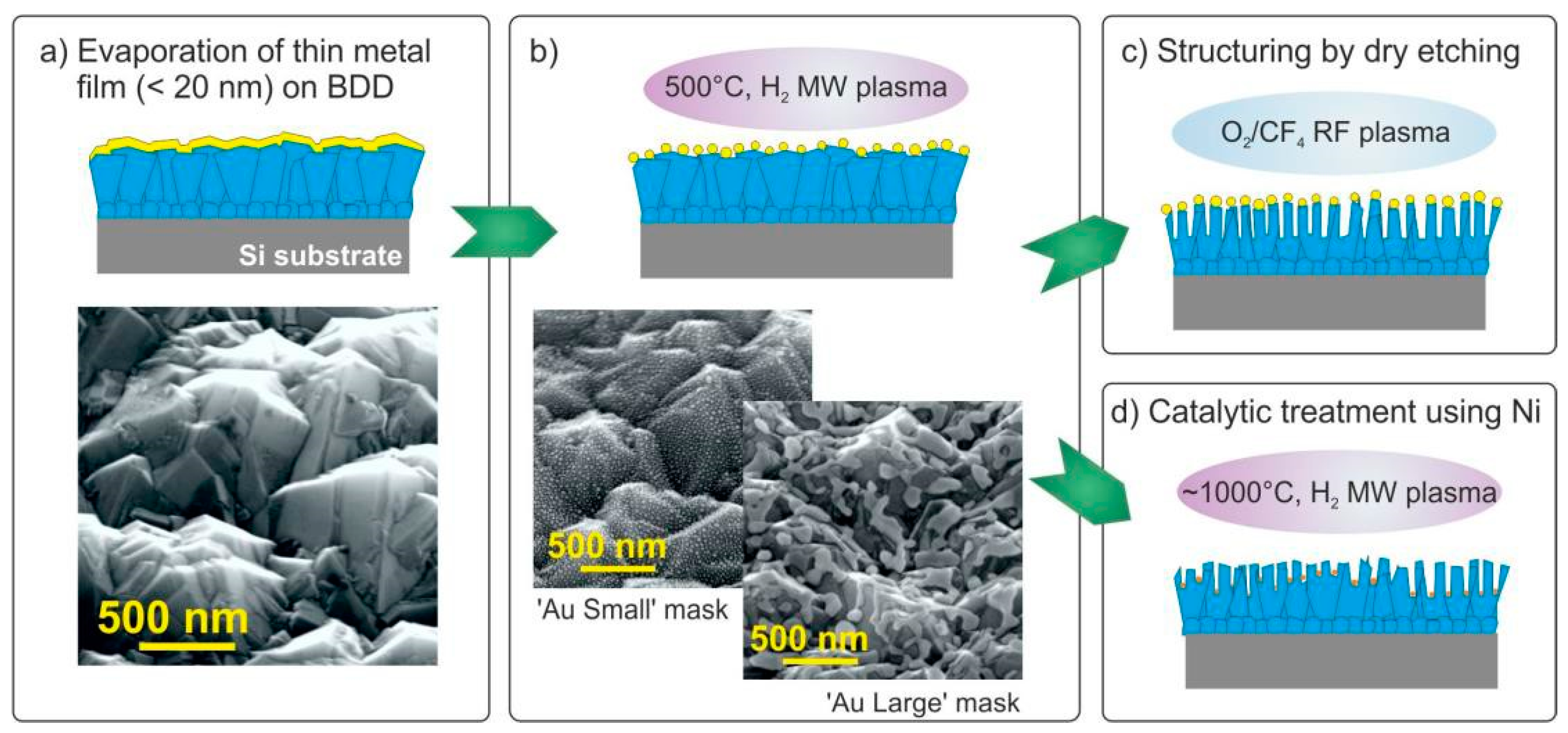
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sahoo, P.K.; Panigrahy, B.; Sahoo, S.; Satpati, A.K.; Li, D.; Bahadur, D. In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: As an electroactive material for analysis of heavy metals. Biosens. Bioelectron. 2013, 43, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Singh, P.; Singh, J.; Sachan, S.; Srivastava, S.; Singh, S.K. Nanocarbon-based Electrochemical Detection of Heavy Metals. Electroanalysis 2016, 28, 2472–2488. [Google Scholar] [CrossRef]
- Le, T.S.; Da Costa, P.; Huguet, P.; Sistat, P.; Pichot, F.; Silva, F.; Renaud, L.; Cretin, M. Upstream microelectrodialysis for heavy metals detection on boron doped diamond. J. Electroanal. Chem. 2012, 670, 50–55. [Google Scholar] [CrossRef]
- Prado, C.; Wilkins, S.J.; Marken, F.; Compton, R.G. Simultaneous Electrochemical Detection and Determination of Lead and Copper at Boron-Doped Diamond Film Electrodes. Electroanalysis 2002, 14, 262–272. [Google Scholar] [CrossRef]
- McGaw, E.A.; Swain, G.M. A comparison of boron-doped diamond thin-film and Hg-coated glassy carbon electrodes for anodic stripping voltammetric determination of heavy metal ions in aqueous media. Anal. Chim. Acta 2006, 575, 180–189. [Google Scholar] [CrossRef] [PubMed]
- El Tall, O.; Jaffrezic-Renault, N.; Sigaud, M.; Vittori, O. Anodic Stripping Voltammetry of Heavy Metals at Nanocrystalline Boron-Doped Diamond Electrode. Electroanalysis 2007, 19, 1152–1159. [Google Scholar] [CrossRef]
- Dragoe, D.; Spătaru, N.; Kawasaki, R.; Manivannan, A.; Spătaru, T.; Tryk, D.A.; Fujishima, A. Detection of trace levels of Pb2+ in tap water at boron-doped diamond electrodes with anodic stripping voltammetry. Electrochim. Acta 2006, 51, 2437–2441. [Google Scholar] [CrossRef]
- Ižák, T.; Domonkos, M.; Babchenko, O.; Varga, M.; Rezek, B.; Jurka, V.; Hruška, K.; Kromka, A. Technological Aspects in Fabrication of Micro- and Nano-Sized Carbon Based Features: Nanorods, Periodical Arrays and Self-Standing Membranes. J. Electr. Eng. 2015, 66, 282–286. [Google Scholar] [CrossRef]
- Babchenko, O.; Kromka, A.; Hruska, K.; Michalka, M.; Potmesil, J.; Vanecek, M. Nanostructuring of diamond films using self-assembled nanoparticles. Open Phys. 2009, 7, 310–314. [Google Scholar] [CrossRef]
- Uetsuka, H.; Yamada, T.; Shikata, S. ICP etching of polycrystalline diamonds: Fabrication of diamond nano-tips for AFM cantilevers. Diam. Relat. Mater. 2008, 17, 728–731. [Google Scholar] [CrossRef]
- Izak, T.; Kromka, A.; Babchenko, O.; Ledinsky, M.; Hruska, K.; Verveniotis, E. Comparative study on dry etching of polycrystalline diamond thin films. Vacuum 2012, 86, 799–802. [Google Scholar] [CrossRef]
- Romanyuk, O.; Varga, M.; Tulic, S.; Izak, T.; Jiricek, P.; Kromka, A.; Skakalova, V.; Rezek, B. Study of Ni-Catalyzed Graphitization Process of Diamond by in Situ X-ray Photoelectron Spectroscopy. J. Phys. Chem. C 2018, 122, 6629–6636. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ižák, T.; Szabó, O.; Štenclová, P.; Potocký, Š.; Vyskočil, V.; Kromka, A. Fabrication of Structured Boron-Doped Diamond Films for Electrochemical Applications. Proceedings 2018, 2, 984. https://doi.org/10.3390/proceedings2130984
Ižák T, Szabó O, Štenclová P, Potocký Š, Vyskočil V, Kromka A. Fabrication of Structured Boron-Doped Diamond Films for Electrochemical Applications. Proceedings. 2018; 2(13):984. https://doi.org/10.3390/proceedings2130984
Chicago/Turabian StyleIžák, Tibor, Ondrej Szabó, Pavla Štenclová, Štěpán Potocký, Vlastimil Vyskočil, and Alexander Kromka. 2018. "Fabrication of Structured Boron-Doped Diamond Films for Electrochemical Applications" Proceedings 2, no. 13: 984. https://doi.org/10.3390/proceedings2130984
APA StyleIžák, T., Szabó, O., Štenclová, P., Potocký, Š., Vyskočil, V., & Kromka, A. (2018). Fabrication of Structured Boron-Doped Diamond Films for Electrochemical Applications. Proceedings, 2(13), 984. https://doi.org/10.3390/proceedings2130984





