Integration of Microresonant Sensor into a Microfluidic Platform for the Real Time Analysis of Platelets-Collagen Interaction in Flow Condition †
Abstract
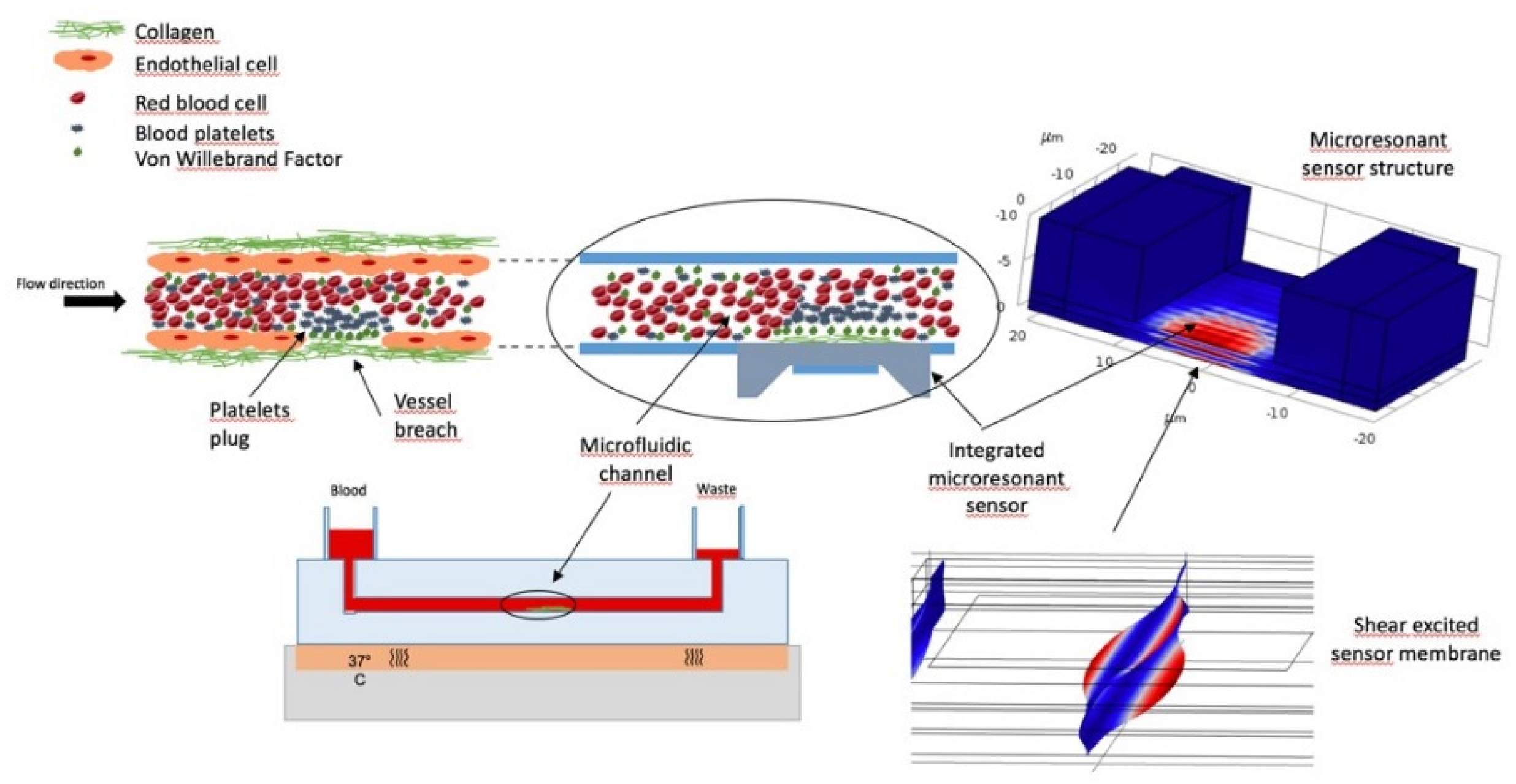
:1. Introduction
2. Materials and Methods
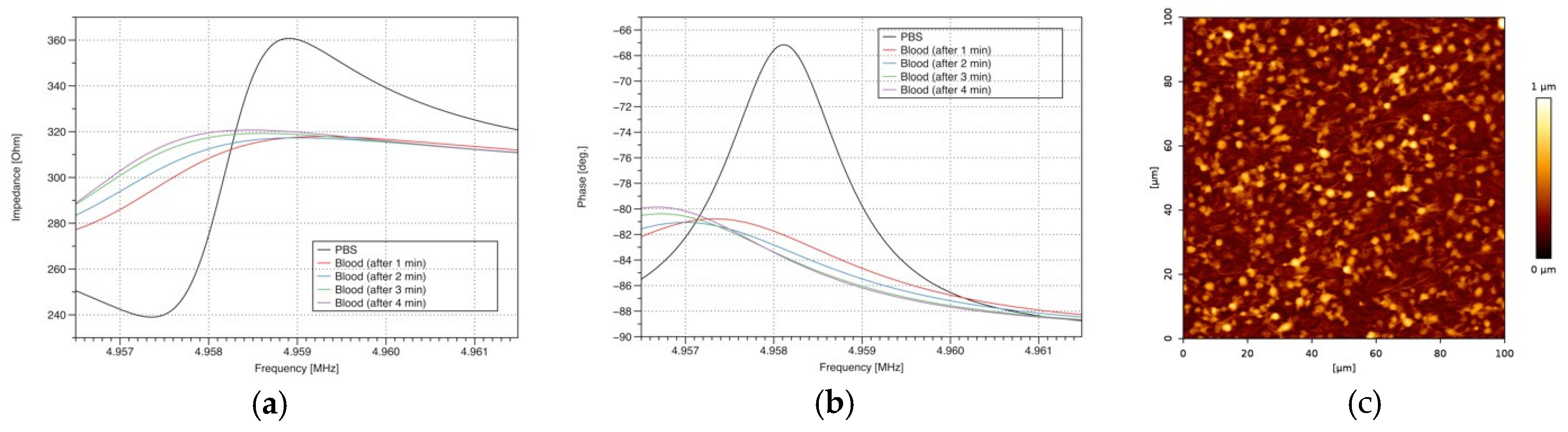
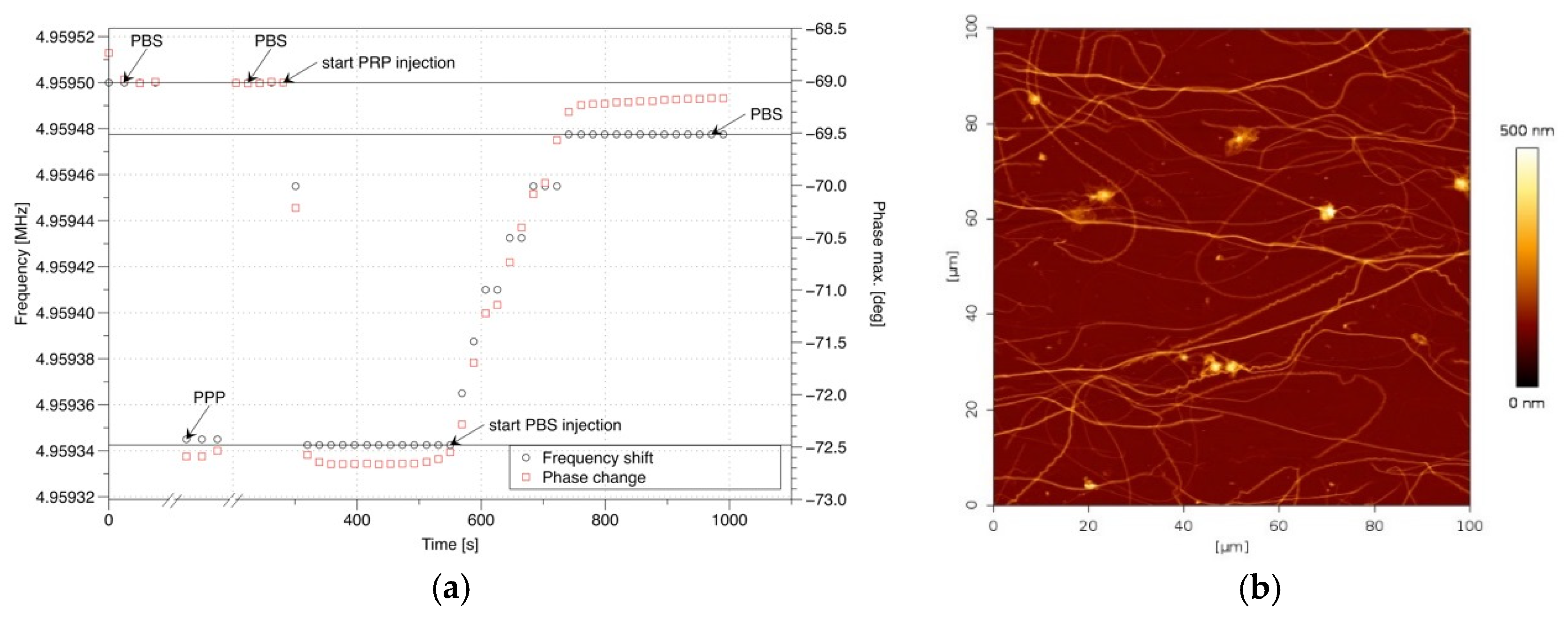
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCarty, O.J.T.; Ku, D.; Sugimoto, M.; King, M.R.; Cosemans, J.M.E.M.; Neeves, K.B. Dimensional analysis and scaling relevant to flow models of thrombus formation: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Albers, W.M.; Tuppurainen, J.; Link, M.; Gabl, R.; Wersing, W.; Schreiter, M. Shear mode FBARs as highly sensitive liquid biosensors. Sens. Actuators A Phys. 2006, 128, 84–88. [Google Scholar] [CrossRef]
- Leblois, T.G.; Tellier, C.R.; Bourquin, R. The quality factor of deeply etched quartz resonators: Theory and experiments. Rev. Phys. Appl. 1989, 24, 877–892. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G., II. The oscillation frequency of a quartz resonator in contact with liquid. Anal. Chim. Acta 1985, 175, 99–105. [Google Scholar] [CrossRef]
- Sauerbrey, G.Z. The use of quartz oscillators for weighing thin layers and for microweighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oseev, A.; Boiseaumarié, B.L.R.d.; Remy-Martin, F.; Manceau, J.-F.; Rouleau, A.; Chollet, F.; Boireau, W.; Leblois, T. Integration of Microresonant Sensor into a Microfluidic Platform for the Real Time Analysis of Platelets-Collagen Interaction in Flow Condition. Proceedings 2018, 2, 940. https://doi.org/10.3390/proceedings2130940
Oseev A, Boiseaumarié BLRd, Remy-Martin F, Manceau J-F, Rouleau A, Chollet F, Boireau W, Leblois T. Integration of Microresonant Sensor into a Microfluidic Platform for the Real Time Analysis of Platelets-Collagen Interaction in Flow Condition. Proceedings. 2018; 2(13):940. https://doi.org/10.3390/proceedings2130940
Chicago/Turabian StyleOseev, Aleksandr, Benoît Le Roy de Boiseaumarié, Fabien Remy-Martin, Jean-François Manceau, Alain Rouleau, Franck Chollet, Wilfrid Boireau, and Thérèse Leblois. 2018. "Integration of Microresonant Sensor into a Microfluidic Platform for the Real Time Analysis of Platelets-Collagen Interaction in Flow Condition" Proceedings 2, no. 13: 940. https://doi.org/10.3390/proceedings2130940
APA StyleOseev, A., Boiseaumarié, B. L. R. d., Remy-Martin, F., Manceau, J.-F., Rouleau, A., Chollet, F., Boireau, W., & Leblois, T. (2018). Integration of Microresonant Sensor into a Microfluidic Platform for the Real Time Analysis of Platelets-Collagen Interaction in Flow Condition. Proceedings, 2(13), 940. https://doi.org/10.3390/proceedings2130940







