Impedimetric Characterization of Interdigitated Electrode Arrays for Biosensor Applications †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Manufacturing and Functionalization of Gold IDAs
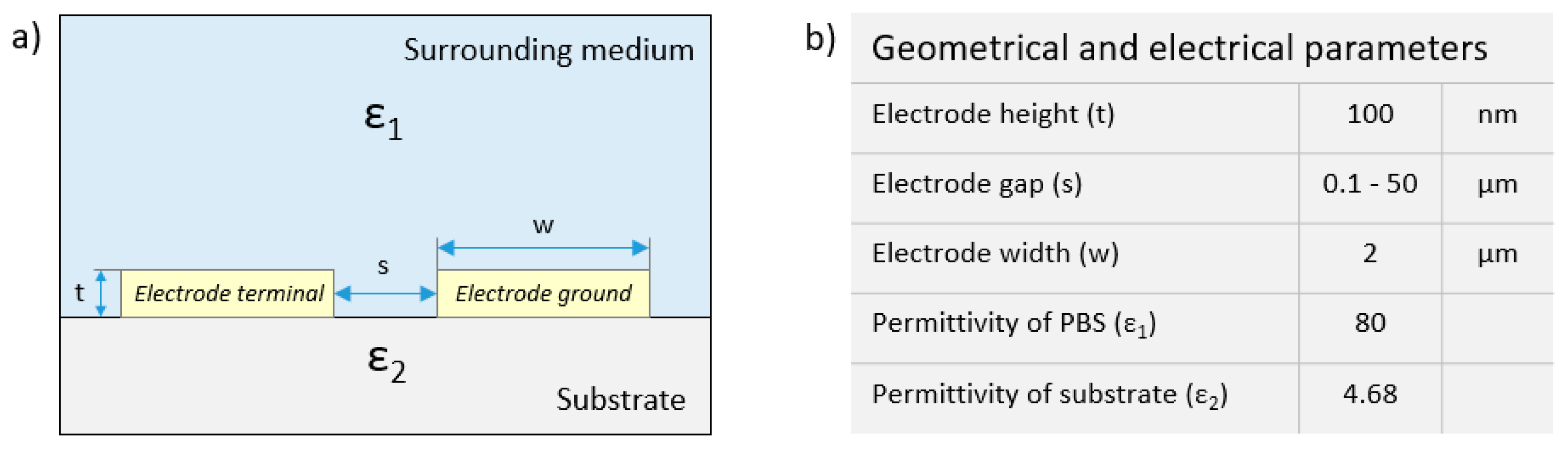
2.3. FEM Simulations of the Electric Field Strength
2.4. Electrochemical Impedance Spectroscopy of Gold IDAs
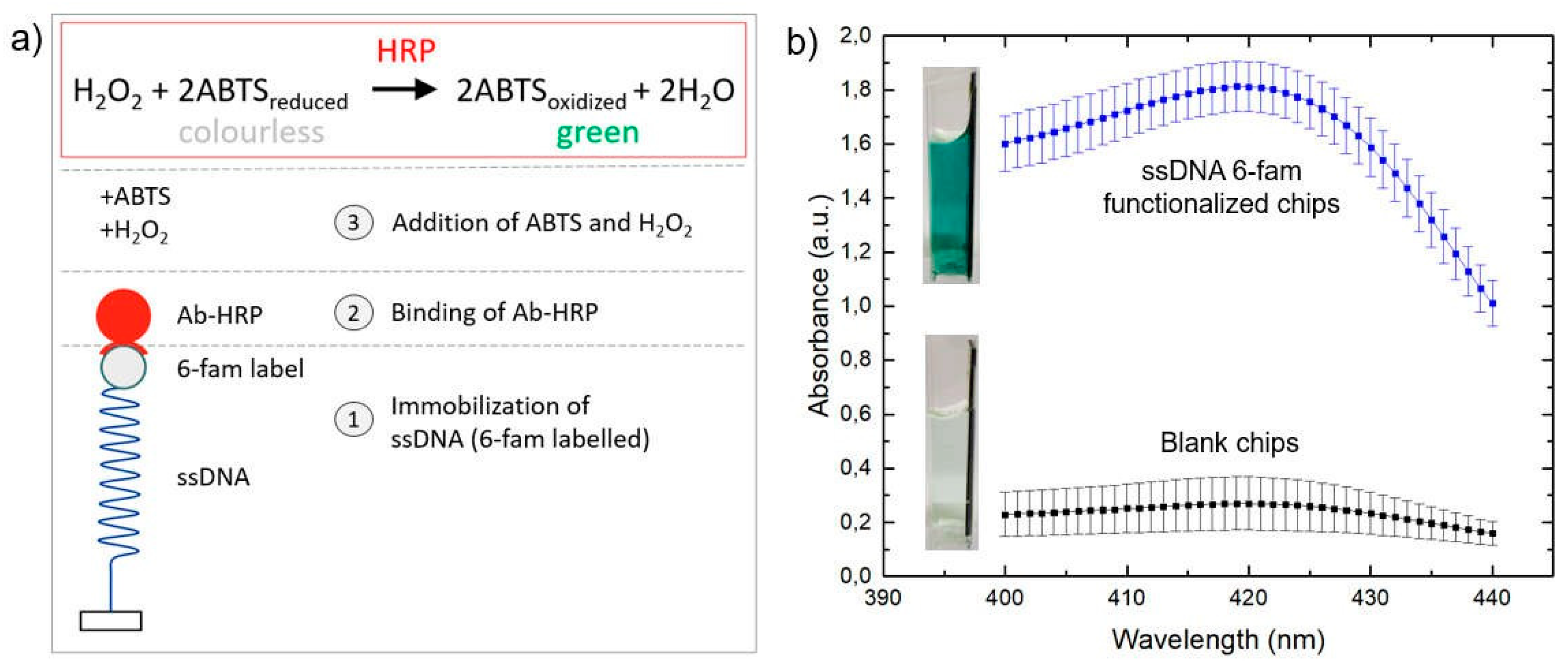
2.5. On-Chip Enzyme Linked Immunosorption Assay (On-Chip ELISA)
3. Results and Discussion
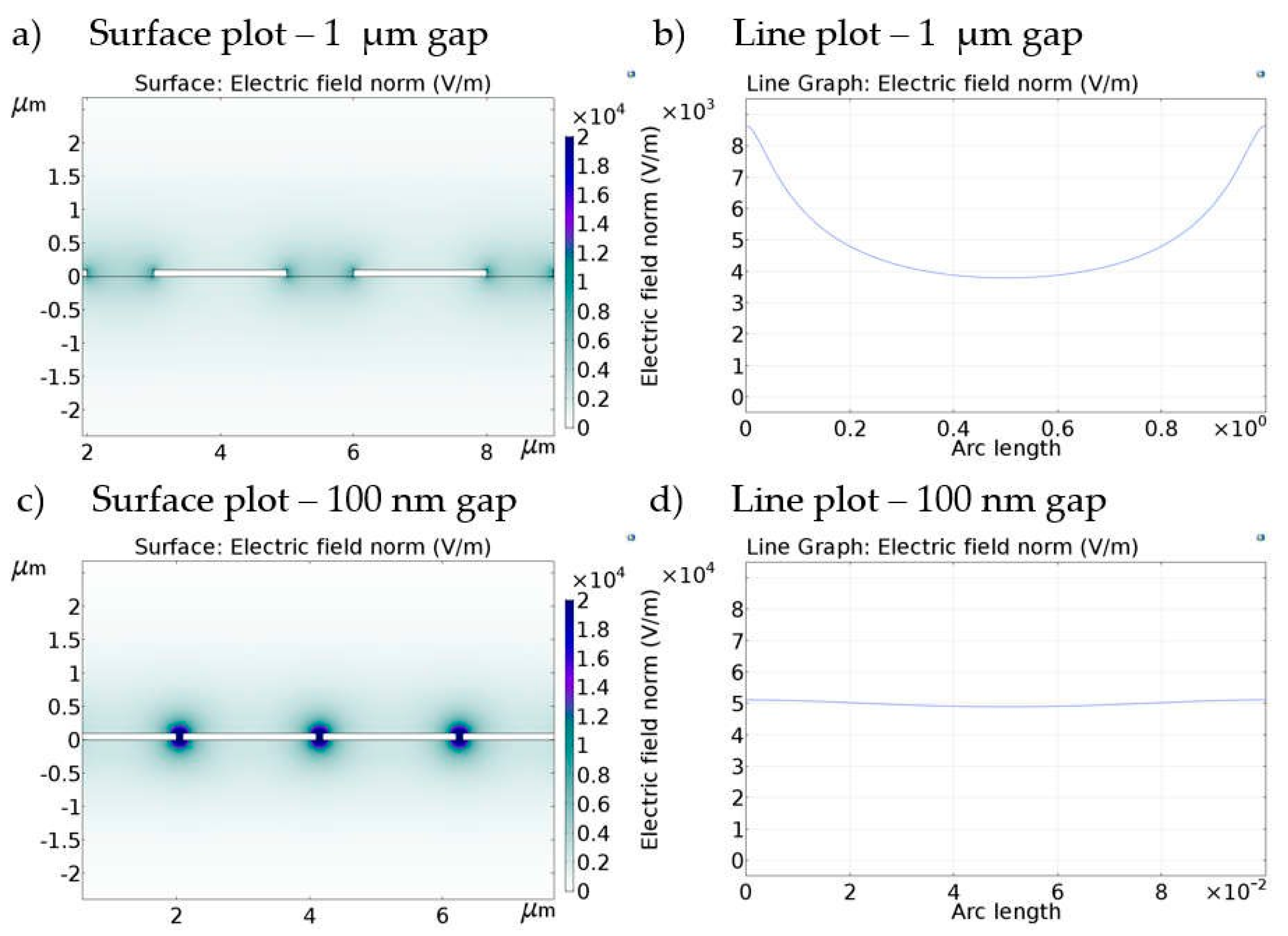
3.1. FEM Simulations
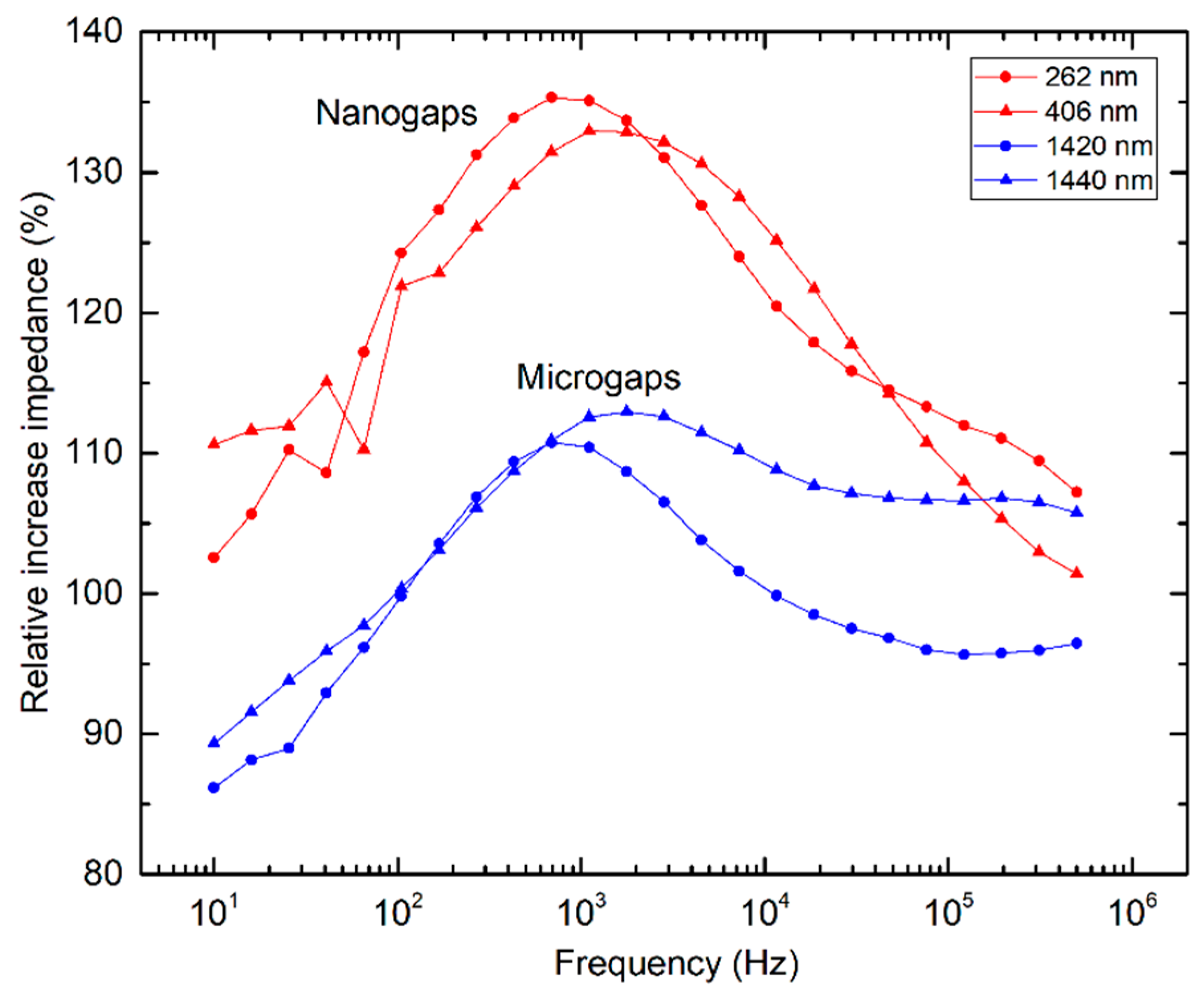
3.2. Impedimetric Characterization
3.3. On-Chip ELISA
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Y.B.; Yanagimoto, Y.; Uno, S.; Nakazato, K. Electrical characteristics of biomodified electrodes using nonfaradaic electrochemical impedance spectroscopy. World Acad. Sci. Eng. Technol. 2011, 73, 295–299. [Google Scholar]
- Singh, K.V.; Whited, A.M.; Ragineni, Y.; Barrett, T.W.; King, J.; Solanki, R. 3D nanogap interdigitated electrode array biosensors. Anal. Bioanal. Chem. 2010, 397, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Partel, S.; Dincer, C.; Kasemann, S.; Kieninger, J.; Edlinger, J.; Urban, G. Lift-Off Free Fabrication Approach for Periodic Structures with Tunable Nano Gaps for Interdigitated Electrode Arrays. ACS Nano 2016, 10, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostal, E.; Kasemann, S.; Dincer, C.; Partel, S. Impedimetric Characterization of Interdigitated Electrode Arrays for Biosensor Applications. Proceedings 2018, 2, 899. https://doi.org/10.3390/proceedings2130899
Kostal E, Kasemann S, Dincer C, Partel S. Impedimetric Characterization of Interdigitated Electrode Arrays for Biosensor Applications. Proceedings. 2018; 2(13):899. https://doi.org/10.3390/proceedings2130899
Chicago/Turabian StyleKostal, Elisabeth, Stephan Kasemann, Can Dincer, and Stefan Partel. 2018. "Impedimetric Characterization of Interdigitated Electrode Arrays for Biosensor Applications" Proceedings 2, no. 13: 899. https://doi.org/10.3390/proceedings2130899
APA StyleKostal, E., Kasemann, S., Dincer, C., & Partel, S. (2018). Impedimetric Characterization of Interdigitated Electrode Arrays for Biosensor Applications. Proceedings, 2(13), 899. https://doi.org/10.3390/proceedings2130899





