Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes †
Abstract
1. Introduction
2. Materials and Methods
2.1. Microscopy Setup
2.2. Image Reconstruction and Examination
2.3. Samples
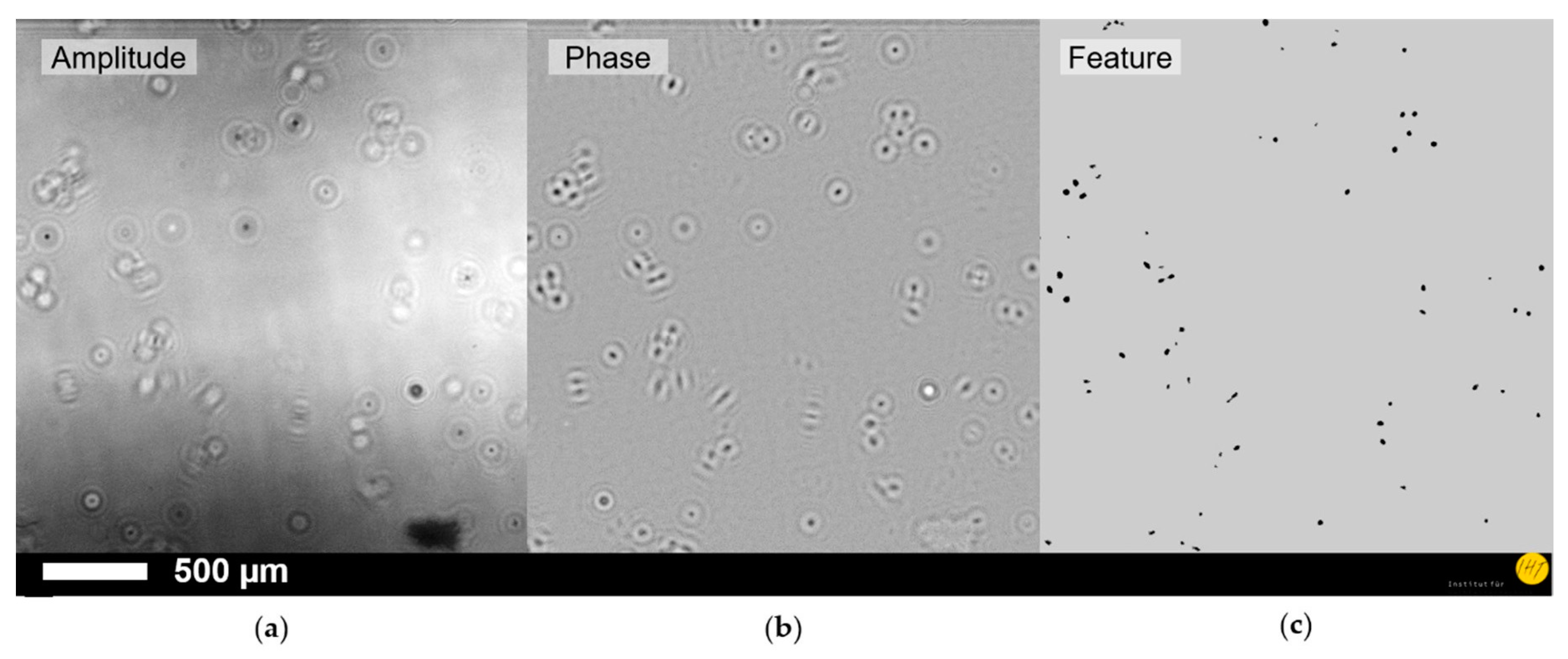
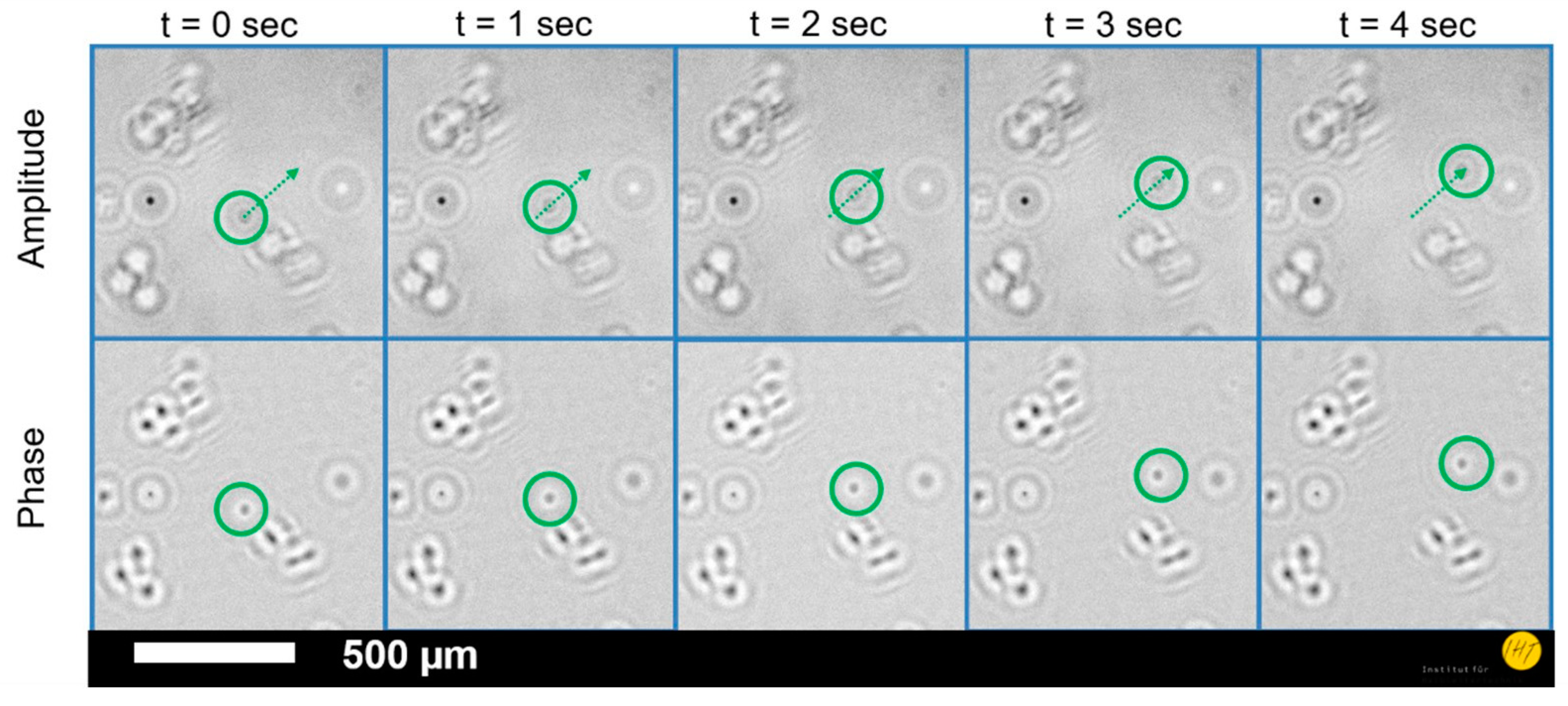
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Henley, B.M.; Kim, C.H.; Lester, H.A.; Yang, C. Incubator embedded cell culture imaging system (EmSight) based on Fourier ptychographic microscopy. Biomed. Opt. Express 2016, 7, 3097. [Google Scholar] [CrossRef] [PubMed]
- Dosch, J.; Hadley, E.; Wiese, C.; Soderberg, M.; Houwman, T.; Ding, K.; Kharazova, A.; Collins, J.L.; van Knippenberg, B.; Gregory, C.; et al. Time-lapse microscopic observation of non-dividing cells in cultured human osteosarcoma MG-63 cell line. Cell Cycle 2018, 17, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Scholz, G.; Xu, Q.; Schulze, T.; Boht, H.; Mattern, K.; Hartmann, J.; Dietzel, A.; Scherneck, S.; Rustenbeck, I.; Prades, J.; et al. LED-Based Tomographic Imaging for Live-Cell Monitoring of Pancreatic Islets in Microfluidic Channels. Proceedings 2017, 1, 552. [Google Scholar] [CrossRef]
- Latychevskaia, T.; Fink, H.-W. Practical algorithms for simulation and reconstruction of digital in-line holograms. Appl. Opt. 2014, 54, 2424–34. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, S. Characterization of mouse cell line IMA 2.1 as a potential model system to study astrocyte functions. ALTEX 2012, 29, 261–274. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, G.; Mariana, S.; Syamsu, I.; Dharmawan, A.B.; Schulze, T.; Mattern, K.; Hörmann, P.; Hartmann, J.; Dietzel, A.; Rustenbeck, I.; et al. Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes. Proceedings 2018, 2, 877. https://doi.org/10.3390/proceedings2130877
Scholz G, Mariana S, Syamsu I, Dharmawan AB, Schulze T, Mattern K, Hörmann P, Hartmann J, Dietzel A, Rustenbeck I, et al. Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes. Proceedings. 2018; 2(13):877. https://doi.org/10.3390/proceedings2130877
Chicago/Turabian StyleScholz, Gregor, Shinta Mariana, Iqbal Syamsu, Agus Budi Dharmawan, Torben Schulze, Kai Mattern, Philipp Hörmann, Jana Hartmann, Andreas Dietzel, Ingo Rustenbeck, and et al. 2018. "Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes" Proceedings 2, no. 13: 877. https://doi.org/10.3390/proceedings2130877
APA StyleScholz, G., Mariana, S., Syamsu, I., Dharmawan, A. B., Schulze, T., Mattern, K., Hörmann, P., Hartmann, J., Dietzel, A., Rustenbeck, I., Hiller, K., Prades, J. D., Waag, A., & Wasisto, H. S. (2018). Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes. Proceedings, 2(13), 877. https://doi.org/10.3390/proceedings2130877







