Abstract
In this paper we present the first results of our research regarding microwave biosensors for non-invasive permittivity monitoring of the human body. To detect permittivity changes, resonators are used, which alter their resonance frequency and their quality factor depending on the permittivity of the material they are placed upon. These permittivity changes may be used as indicators for the heart rate, blood pressure or glucose level. As a proof of concept, this paper focusses on designing resonators to detect the heart beat of humans in the Radial Artery, which is located in the forearm.
1. Motivation and Theory
Illnesses in the cardiovascular system usually require periodic monitoring. Patients of some of these illnesses are advised to check their symptoms daily, in special cases even multiple times per day. These measurements can at least be a hassle and for invasive monitoring techniques even painful.
In this research we focus on developing resonators, which could help in monitoring some symptoms non-invasively. Since blood is the dominant part in human tissues in regards to the permittivity at low gigahertz frequencies (<10 GHz), the detected changes may be useful for future continuous monitoring systems, especially for heart rate, glucose concentration and blood pressure measurements.
Microwave frequencies enable the design of small and cheap resonators consisting of gaps as capacitances and lines as inductances. In this case the structures can be designed to be even smaller than usual, since the high permittivity of the human tissue, which is placed on top of the resonator, effectively lower the wavelength. Because of the small size and the high quality factor, split-ring resonators are used in this research work.
Gaps acting as capacitances in microwave resonators are highly sensitive to permittivity changes [1]. In theory this makes it possible to detect the volume change of blood in human arteries and thus enables the design of a potentially wearable heart rate or blood pressure sensor. In [2] other studies were conducted on the permittivity changes of varying glucose concentrations in blood and water, which state that a similar measurement could be possible to detect the amount of glucose in the blood. In this work, we focus on the general concept of designing the said resonators with their use as biosensors in mind.
2. Simulations in CST Microwave Studio
To maximize the quality factor of the resonators, the first simulations in CST Microwave Studio contained the Cole-Cole Material “Blood” [3] on top of the resonator structure. This enabled a fast optimization. Two of the designed resonators are shown in Figure 1a,b.
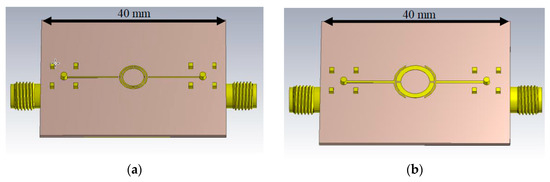
Figure 1.
CST Microwave Studio models of the simulated split-ring resonators. (a) Double split-ring resonator; (b) Symmetrical split-ring resonator.
After optimizing the resonators, they were simulated in conjunction with a 3D human body model, which was obtained from IT’IS [4]. The model is part of the ViP 2.x range, which contains 22 different human tissues. Since it is a highly detailed 3D file, which would take too much time to compute, it was first reduced to only the right forearm. Also, the polygons of this model were reduced to speed up the simulations even further. Both the cutting and the reduction of the model were done with the free CAD Software Autodesk Meshmixer.
To simulate the models as the intended tissues, their material properties in CST Microwave Studio were set to include a manual dispersion list, which was calculated using the Cole-Cole parameters given in [3]. Additionally arteries, veins and tendons were implemented. Since the real resonators will be pressed against the skin, the model was flattened in some areas, to simulate the pressure of the resonator. The resulting model (as seen in Figure 2) is a rough approximation of a human forearm. This approximation will be sufficient, since human anatomy varies slightly from person to person and the Cole-Cole parameters are also fitted to specific measurements.
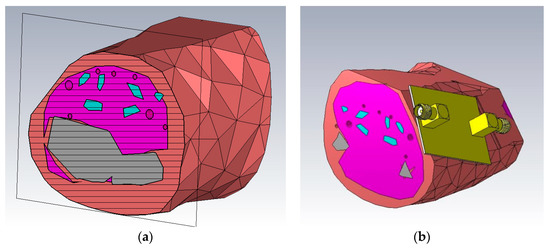
Figure 2.
CST Microwave Studio models of the forearm. The salmon colored material is simulated as skin, the pink colored material represents muscles, grey is used for bones, red for arteries and veins and finally cyan for tendons. (a) Cross section of the model; (b) Resonator placed on the model.
Roughly based on values found in [5] the diameter of the arteries was swept between 2.5 mm and 2.8 mm to determine the impact of the heartrate on the resonance of the microwave structures. As expected, the results in Figure 3 show, that the resonance shifted slightly. The power flow in this model can be seen in Figure 4.
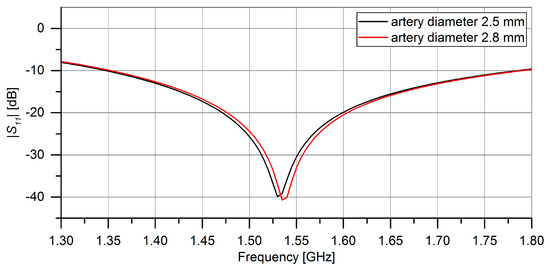
Figure 3.
CST Microwave Studio Simulation results for the impact of different artery diameters on the resonance of the symmetrical split-ring resonator. In black: ∅artery = 2.5 mm. In red: ∅artery = 2.8 mm.
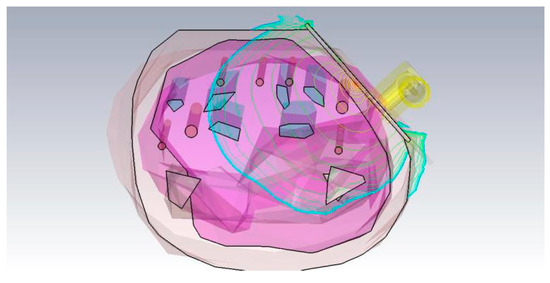
Figure 4.
CST Microwave Studio simulation qualitative power flow in the resonator and the human body model, logarithmically scaled.
3. Measurement Results
After fabricating the resonators with an LPKF milling plotter ProtoMat S63, first measurements were conducted to test if the heart rate was detectable in the forearm above the radial artery. For this, the arm was carefully placed on top of the resonator. By using an Agilent 8753ES S-Parameter Network Analyzer the resonance frequency was found. The change in resonant frequency caused by different pressures was also observed. This way it was possible to keep the pressure more constant and thus minimizing unwanted resonance shifts.
Then the settings were changed to a continuous wave sweep using the obtained resonance frequency. The sweep time was set to 5 s at first to check whether or not a heart rate was detectable. If it wasn’t, the resonator was moved to a slightly different spot on the forearm and the procedure was repeated. Once a systematic pulse was found, the sweep time was increased to 10 s. These measurements were conducted for the magnitude and for the phase of the reflection coefficients S11 or S22. The results of two measurements can be seen in Figure 5a,b.
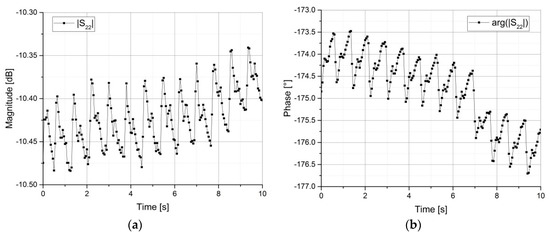
Figure 5.
Network Analyzer measurements of the reflection coefficient S22 of the symmetrical split-ring resonator over 10 s. (a) Magnitude in dB; (b) Phase in degrees.
The phase measurements proved to be easier to conduct, because the magnitude changes are small compared to the power resolution of the network analyzer. The peak-to-peak values for the magnitude are less than 0.1 dB and approximately 1.25°, respectively. The equivalent heart rate of these measurements is roughly 75 bpm, which corresponds to a normal value for the person under test.
4. Conclusions
It is possible to detect the human heart rate non-invasively by placing a resonator on top of the skin and measuring the frequency shift, which corresponds to the change of magnitude or the phase shift of the reflection coefficient. In this paper, the phase shift was easier to measure compared to the magnitude shift. Further research should be done in this field, as it is a promising step towards non-invasive medical instruments for cardiovascular illnesses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shafi, K.T.M.; Jha, A.K.; Akhtar, M.J. Improved Planar Resonant RF Sensor for Retrieval of Permittivity and Permeability of Materials. IEEE Sens. J. 2017, 11, 5479–5486. [Google Scholar] [CrossRef]
- Topsakal, E.; Karacolak, T.; Moreland, E.C. Glucose-Dependent Dielectric Properties of Blood Plasma. In Proceedings of the XXXth URSI General Assembly and Scientific Symposium, Istanbul, Turkey, 13–20 August 2011. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.-C.; Neufeld, E.; Moser, H.; Huber, E.; Farcito, S.; Gerber, L.; Jedensjo, M.; Hilber, I.; di Gennaro, F.; Lloyd, B.; et al. Development of a New Generation of High-Resolution Anatomical Models for Medical Device Evaluation: The Virtual Population 3.0. Phys. Med. Biol. 2014, 59, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; Scandale, G.; Jun, T.; Mlnola, M.; Recchia, M.; Annoni, M. Radial artery compliance in patients with peripheral vascular disease. Vasc. Med. 1997, 2, 8–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).