WO3 Based Gas Sensors †
Abstract
:1. Introduction
2. Experimental
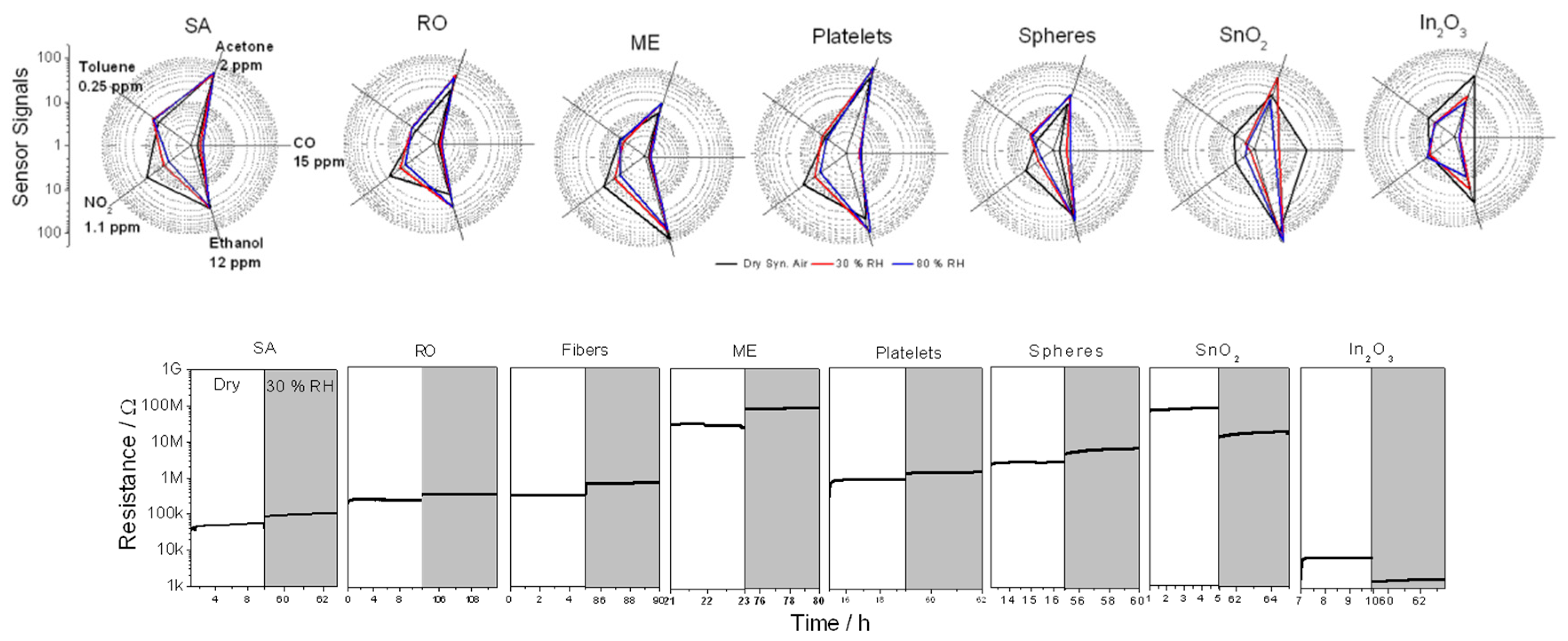
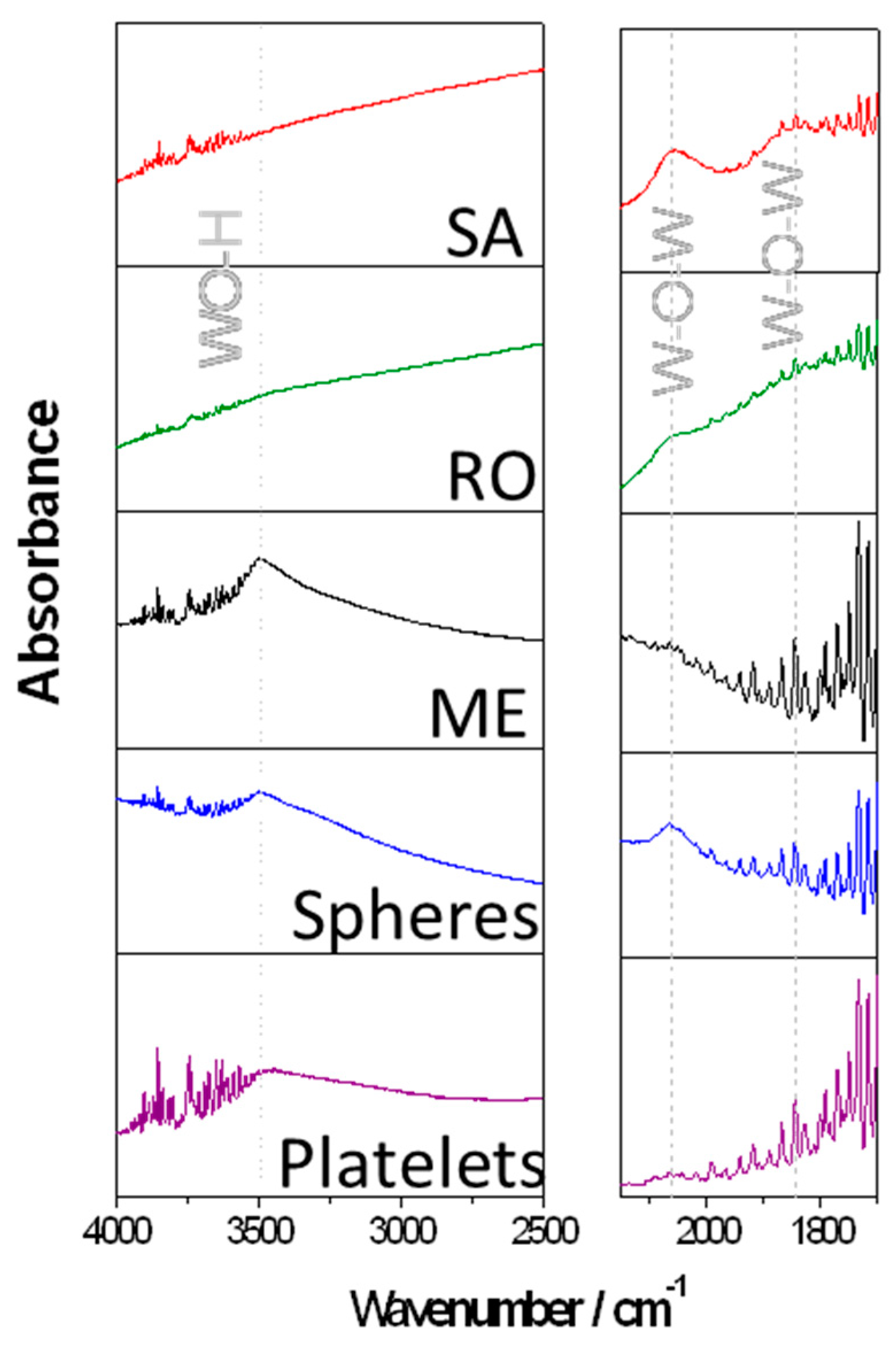
3. Results and Discussion
Author Contributions
References
- Wang, C.; Sun, R.; Li, X.; Sun, Y.; Sun, P.; Liu, F.; Lu, G. Hierarchical flower-like WO3 nanostructures and their gas sensing properties. Sens. Actuators B Chem. 2014, 204, 224–230. [Google Scholar] [CrossRef]
- Penza, M.; Martucci, C.; Cassano, G. NOx gas sensing characteristics of WO3 thin films activated by noble metals (Pd, Pt, Au) layers. Sens. Actuators B Chem. 1998, 50, 52–59. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, I.; Jang, B.H.; Youn, D.Y.; Ryu, W.H.; Park, C.O.; Kim, I.D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.; Park, S.; Lee, C. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B Chem. 2015, 209, 180–185. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kang, Y.C.; Lee, J.H. Highly selective and sensitive detection of trimethylamine using wo3 hollow spheres prepared by ultrasonic spray pyrolysis. Sens. Actuators B Chem. 2013, 176, 971–977. [Google Scholar] [CrossRef]
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Kang, Y.C.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Staerz, A.; Kim, T.-H.; Lee, J.-H.; Weimar, U.; Barsan, N. Nanolevel Control of Gas Sensing Characteristics via p–n Heterojunction between Rh2O3 Clusters and WO3 Crystallites. J. Phys. Chem. C 2017, 121, 24701–24706. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Teodorescu, V.S.; Barsan, N.; Weimar, U. Synthesis, Mechanism, and Gas-Sensing Application of Surfactant Tailored Tungsten Oxide Nanostructures. Adv. Funct. Mater. 2009, 19, 1767–1774. [Google Scholar] [CrossRef]
- Epifani, M.; Andreu, T.; Arbiol, J.; Díaz, R.; Siciliano, P.; Morante, J.R. Chloro-alkoxide route to transition metal oxides. Synthesis of WO3 thin films and powders from a tungsten chloro-methoxide. Chem. Mater. 2009, 21, 5215–5221. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T.; Wicker, S.; Weimar, U.; Barsan, N. The Oxidizing Effect of Humidity on WO3 based Sensors. Sens. Actuators B Chem. 2016, 237, 54–58. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staerz, A.; Somacescu, S.; Epifani, M.; Russ, T.; Weimar, U.; Barsan, N. WO3 Based Gas Sensors. Proceedings 2018, 2, 826. https://doi.org/10.3390/proceedings2130826
Staerz A, Somacescu S, Epifani M, Russ T, Weimar U, Barsan N. WO3 Based Gas Sensors. Proceedings. 2018; 2(13):826. https://doi.org/10.3390/proceedings2130826
Chicago/Turabian StyleStaerz, Anna, Simona Somacescu, Mauro Epifani, Tamara Russ, Udo Weimar, and Nicolae Barsan. 2018. "WO3 Based Gas Sensors" Proceedings 2, no. 13: 826. https://doi.org/10.3390/proceedings2130826
APA StyleStaerz, A., Somacescu, S., Epifani, M., Russ, T., Weimar, U., & Barsan, N. (2018). WO3 Based Gas Sensors. Proceedings, 2(13), 826. https://doi.org/10.3390/proceedings2130826




