UV Light Assisted NO2Sensing by SnO2/Graphene Oxide Composite †
Abstract
:1. Introduction
2. Materials and Methods
2.1. SynthesisMethod
2.2. GasTesting

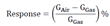
3. Results
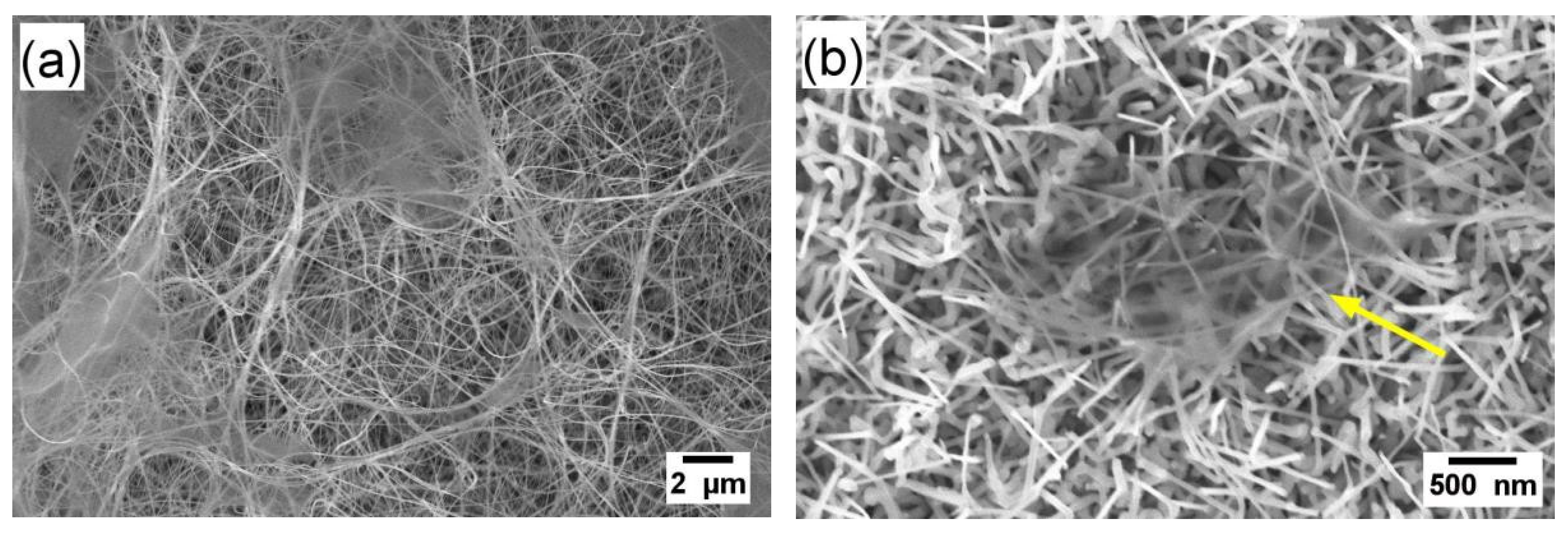
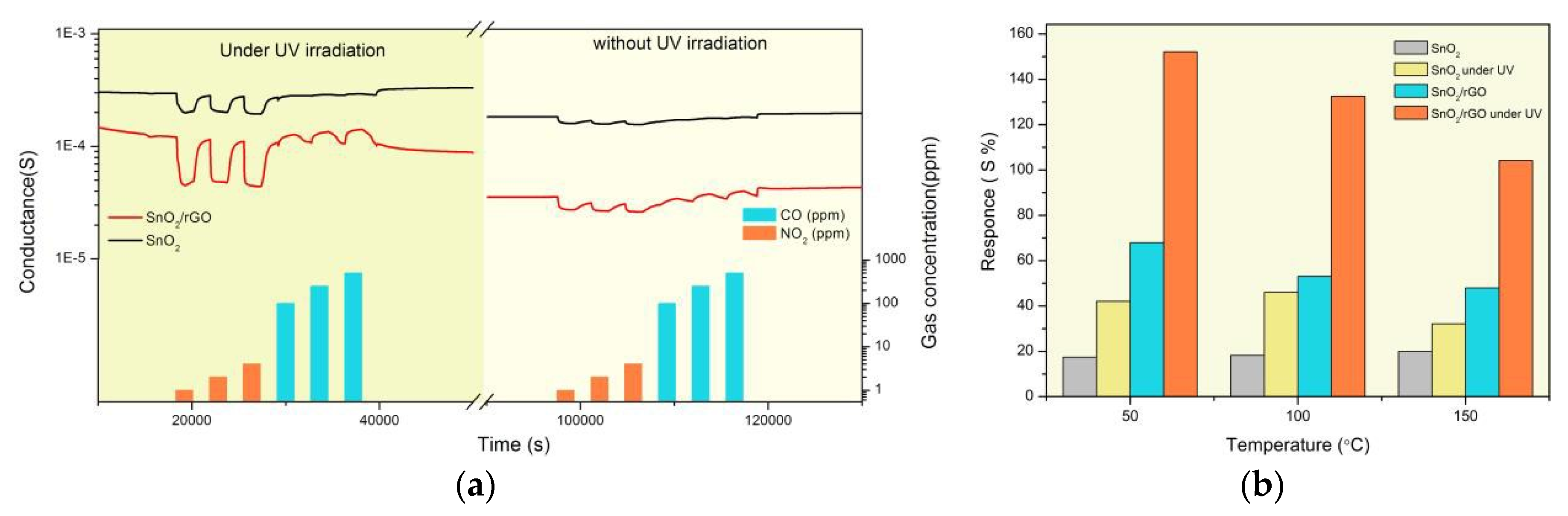
3.1. Characterization and GasTesting
3.2. Gas SensingMechanism
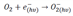
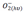 . The chemical reaction between NO2 and oxygen species on the surface explained through the following Equation (4):
. The chemical reaction between NO2 and oxygen species on the surface explained through the following Equation (4):
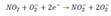
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Geng, X.; Zhang, C.; Olivier, M.; Debliquy, M. Room Temperature NO2 Responses of Visible-Light Activated Nanosheet rGO@ZnO1−x Sensors. Proceedings 2017, 1, 1–5. [Google Scholar]
- Munasinghe, M.A.H.M.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Low Temperature Gas Sensing PropertiesofGrapheneOxide/SnO2NanowiresCompositeforH2. ProcediaEng. 2016, 168, 305–308. [Google Scholar]
- Geng, X.; You, J.; Wang, J.; Zhang, C. Visible Light Assisted Nitrogen Dioxide Sensing Using Tungsten Oxide-Graphene Oxide Nanocomposite Sensors. Mater. Chem. Phys. 2017, 191, 114–120. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D Graphene Aerogel-ZnO Composites as Efficient Detection for NO2at Room Temperature. Sens. Actuators B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Li, J.; Luo, Y.; Lu, P. Role of Oxygen Vacancy in Tuning of Optical, Electrical and NO2sensingPropertiesofZnO1−xcoatingsatRoomTemperature. Sens.ActuatorsBChem. 2017, 248, 886–893. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arachchige, H.M.M.M.; Gunawardhana, N.; Zappa, D.; Comini, E. UV Light Assisted NO2Sensing by SnO2/Graphene Oxide Composite. Proceedings 2018, 2, 787. https://doi.org/10.3390/proceedings2130787
Arachchige HMMM, Gunawardhana N, Zappa D, Comini E. UV Light Assisted NO2Sensing by SnO2/Graphene Oxide Composite. Proceedings. 2018; 2(13):787. https://doi.org/10.3390/proceedings2130787
Chicago/Turabian StyleArachchige, Hashitha M. M. Munasinghe, Nanda Gunawardhana, Dario Zappa, and Elisabetta Comini. 2018. "UV Light Assisted NO2Sensing by SnO2/Graphene Oxide Composite" Proceedings 2, no. 13: 787. https://doi.org/10.3390/proceedings2130787
APA StyleArachchige, H. M. M. M., Gunawardhana, N., Zappa, D., & Comini, E. (2018). UV Light Assisted NO2Sensing by SnO2/Graphene Oxide Composite. Proceedings, 2(13), 787. https://doi.org/10.3390/proceedings2130787





