Abstract
Mixed potential ammonia gas sensors were fabricated by using two sensing materials of Ni3V2O8 and Au-V2O5 as working electrodes, YSZ as electrolyte and platinum as reference electrode. The results have shown that the Ni3V2O8 sensors show cross-sensitivity toward NO gas. However, Au-V2O5 working electrodes displayed a high sensitivity to NH3 as well as fast response and recovery times at high temperatures. Furthermore, the results indicate that the selectivity of Au-V2O5 sensors increases by increasing temperature.
1. Introduction
According to new European driving cycle, over the past years, while the vehicular emissions of NOx and CO have decreased, ammonia emissions have been considerably increased [1]. Today, one of the main research subjects of electrochemical gas sensors to control and monitor automotive exhaust emissions is developing a high-performance single sensor capable of monitoring NOX, NH3, CO, and hydrocarbons at the same time.
Selective Catalytic Reduction (SCR) using ammonia gas (NH3) as a reductant is recognized as the most effective NOX control technology in the world. More recent applications of this system include reduction of NOX emissions generated from diesel engine vehicles and trucks, calcining ovens, gas turbines firing landfill and digester gas. In order to optimize the conversion rates of NOX and to avoid the air pollution of excessive ammonia, an NH3 sensor is required to control the SCR system for maintaining NH3 slip at an acceptable level [2].
Solid-state gas sensing technology is considered one the high effective approaches for gas sensing in harsh environments because of simple structure, good selectivity, high sensitivity and especially excellent stability. In recent years, the development of potentiometric sensors has become quite a revolution in sensor technology for monitoring environmental gases [3,4].
Concerning ammonia gas sensors, vanadium-based materials have been reported as promising materials owing to their catalytic acidic sites caused by vanadium content. Vanadium dioxide (V2O5), have been recognized as sensitive material to NH3 even at ppb levels [5,6].
2. Materials and Methods
In this study, solid-state electrochemical planar sensors were fabricated by screen-printing method using commercial yttria-stabilized zirconia (YSZ), commercial vanadium oxide (V2O5) powder and commercial Au and Pt inks. Homemade inks are elaborated for YSZ and Au-V2O5, by addition of V2O5 to Au ink for the latter.
Ni3V2O8 material was synthesized from nickel nitrate (Ni(NO3)2·6H2O) and ammonium metavanadate (NH4VO3) through sol-gel method [7].
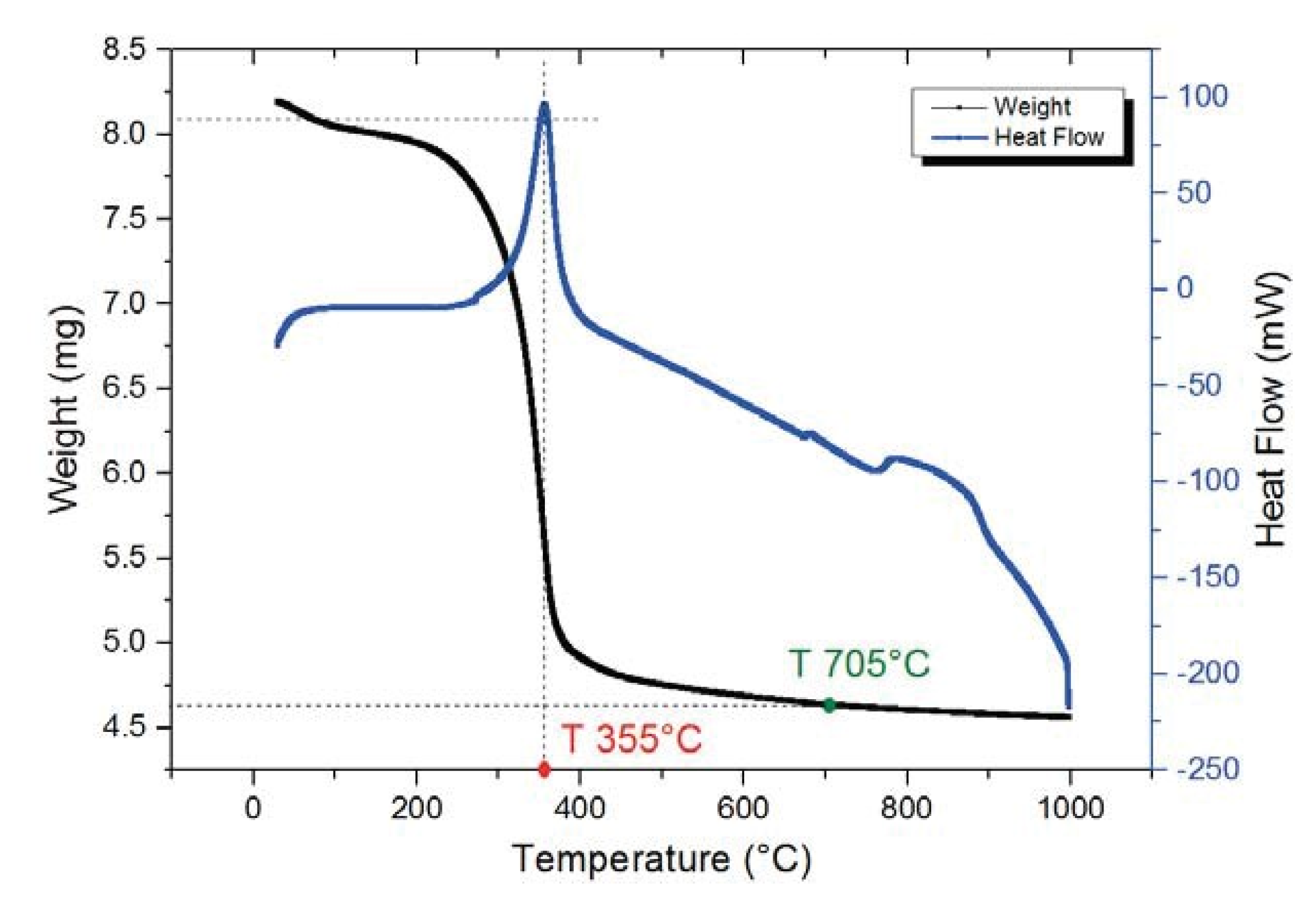
In order to determine the decomposition temperature of the precursors and the more suitable sintering temperature of the obtained composite, thermogravimetric and differential thermal analysis (TGA-DSC) were performed. The DSC profile in Figure 1 reveals a sharp exothermic peak at 355 °C, corresponding to a rapid weight loss in TGA curve. This could indicate the oxidation of organic additives and burning out of the nitrate radicals in the precursor. According to TGA curve, about 44% weight loss takes place before 700 °C. The last exothermic peak, centered approximately at 800 °C, can be due to the crystallization of Ni3V2O8.
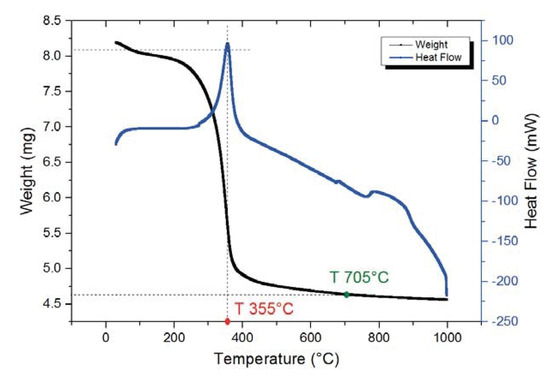
Figure 1.
TGA-DSC analysis of Ni3V2O8 precursor.
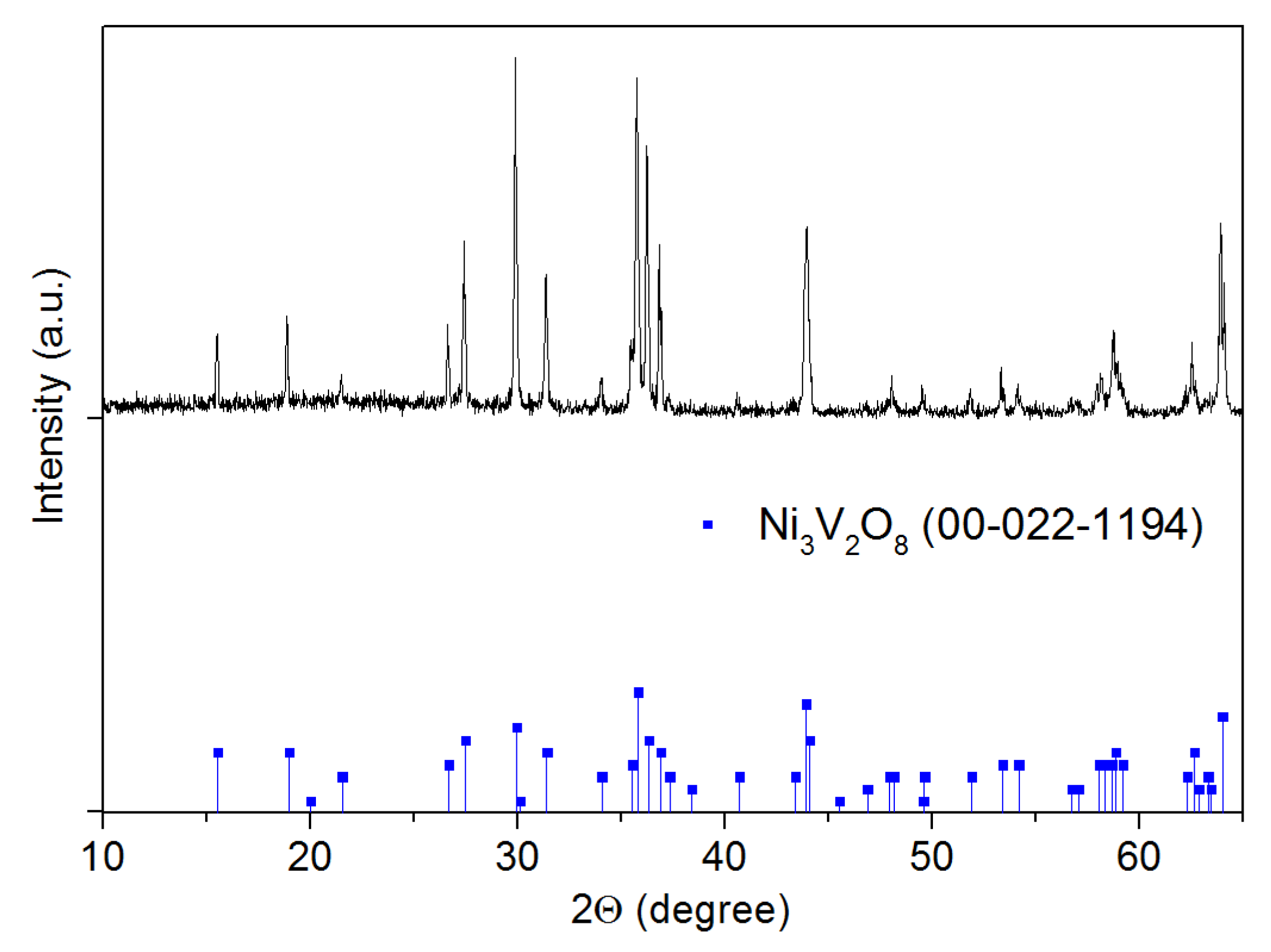
The crystal structure of Ni3V2O8 powder sintered at 1000 °C for 2 h is verified by X-ray diffraction spectrometry (XRD). The used diffractometer is from Siemens company (D5000) equipped with a radiation source of Cu Kα1 + α2. The XRD pattern shown in Figure 2 is obtained with radiation in 8/28 geometry between 10° and 65° with a scan rate of 1 degree/min. The resulted spectrum indicates a single-phase pattern attributed to Ni3V2O8 without any impurity peak.
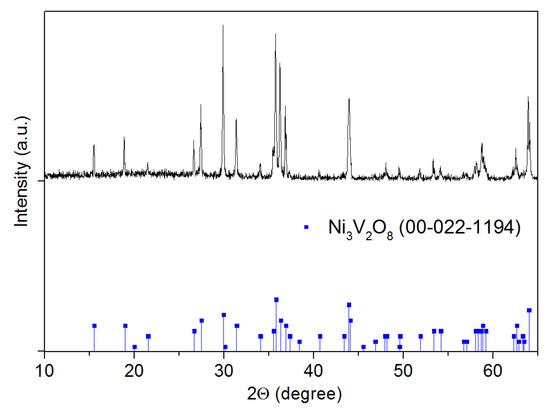
Figure 2.
XRD patterns for Ni3V2O8 powder sintered at 1000 °C for 2 h.
The sensors were then developed on an alumina substrate equipped with a Pt Heater. An YSZ layer (2 × 5 mm2) was first deposited and annealed at 1380 °C. The electrodes, Pt and either Au-V2O5 or Ni3V2O8 were printed on each side of the YSZ layer and connected to gold tracks to measure the sensor signal, ∆V, which is the difference of potential between the reference (Pt) and the working (V-based) electrode. The sintering temperatures were respectively 600, 850, and 1000 °C for Au-V2O5, Pt and Ni3V2O8.
3. Results and Discussion
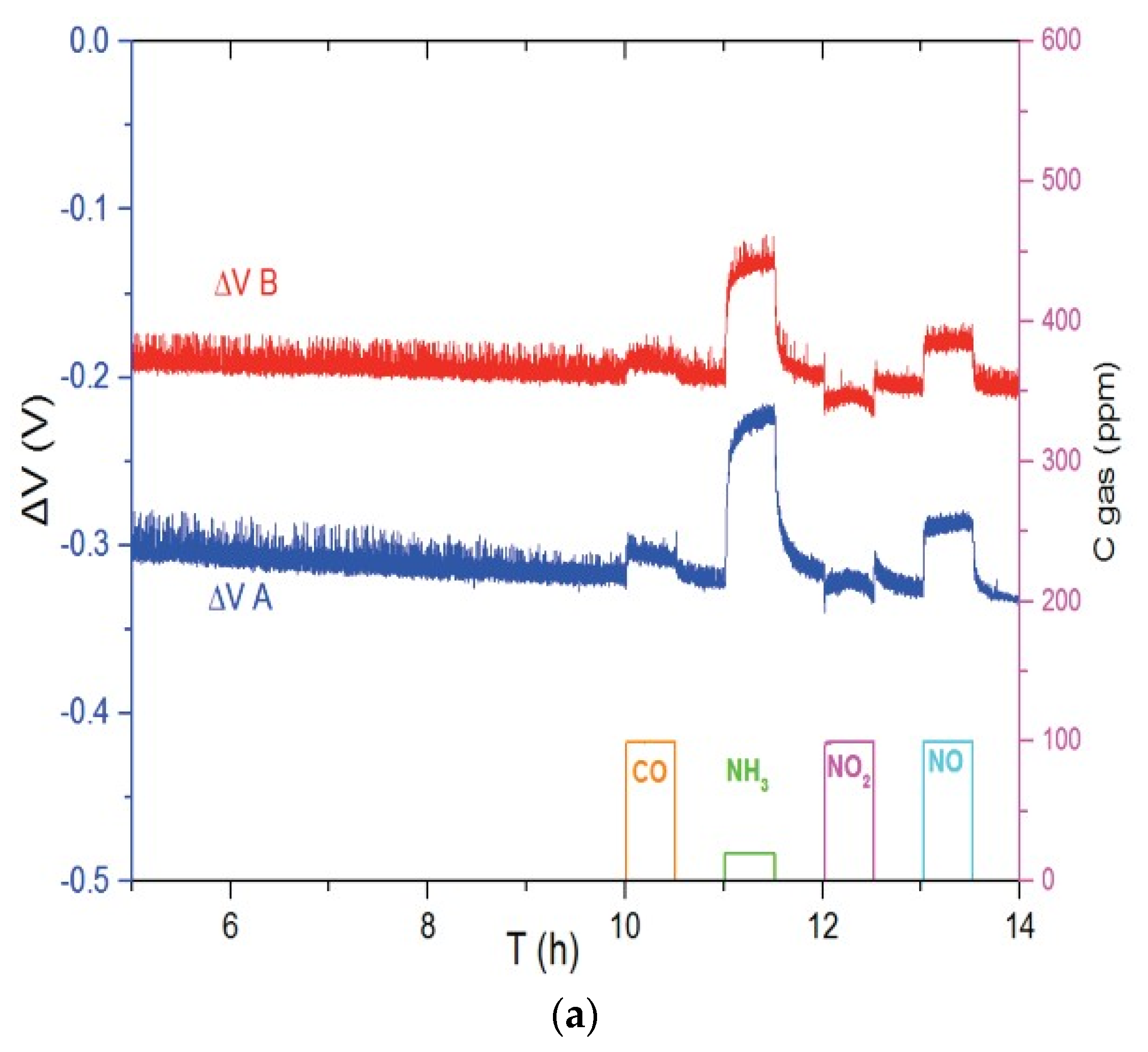
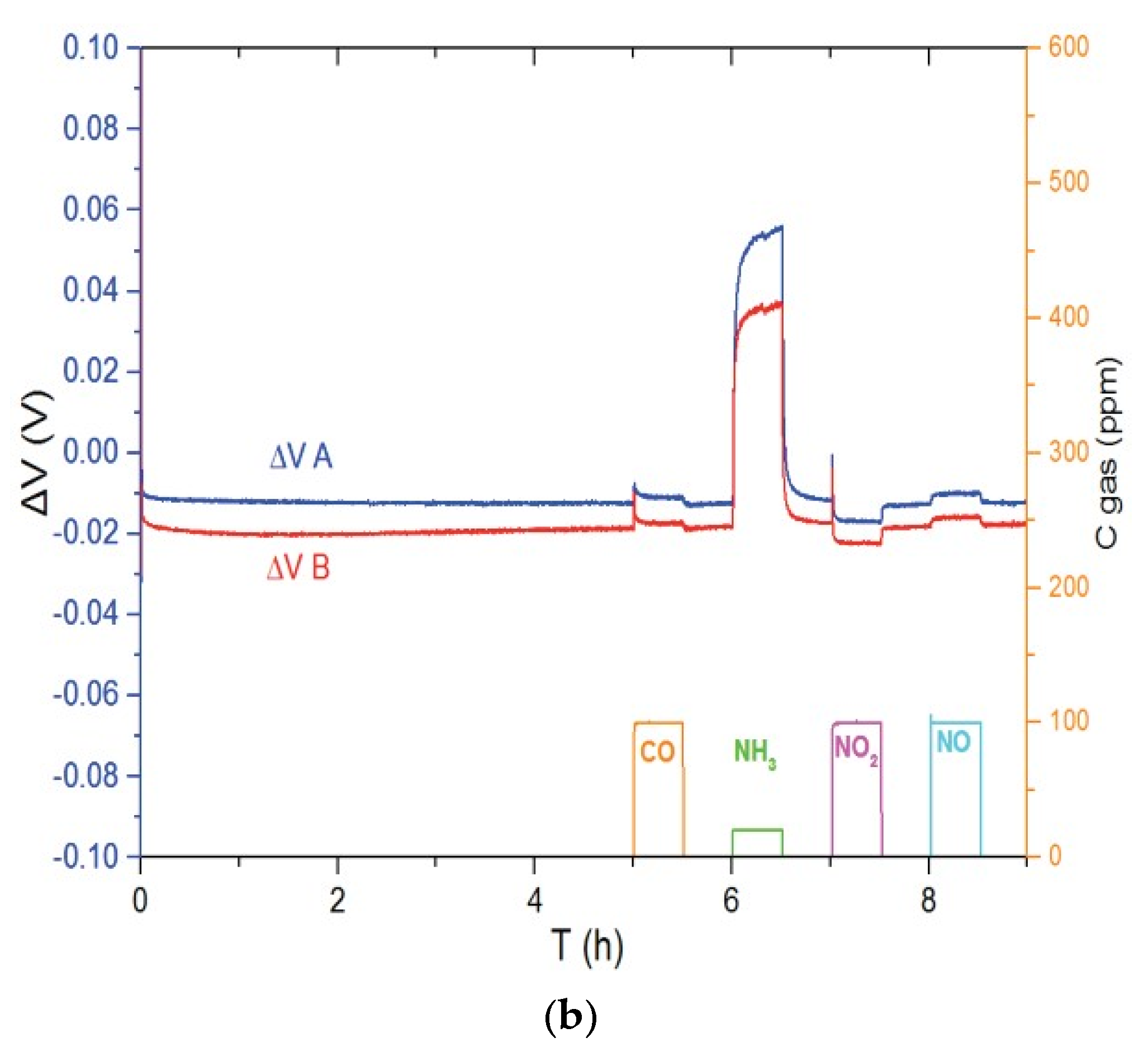
Figure 3 shows the responses of Ni3V2O8 (Figure 3a) and Au-V2O5 (Figure 3b) sensors to different gases such as CO, NH3, NO2 and NO in the base gas of 12% O2 and 1.5% H2O, balanced with N2. In each case, sensors A and B are two sensors which are fabricated and tested at the same conditions in order to take into account irreproducibility of system. It can be seen in Figure 3a, that sensors with Ni3V2O8 working electrode show a response to NH3 at 700 °C but also to NO in lower ratio, while this sensor is weakly sensitive to CO and NO2. Regarding Au-V2O5 electrodes at 600 °C (Figure 3b), it is evident that the sensor is selective to ammonia compared to CO, NO2 and NO gases.
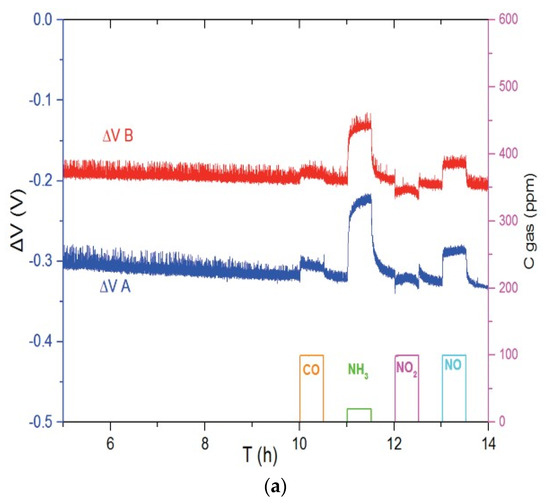
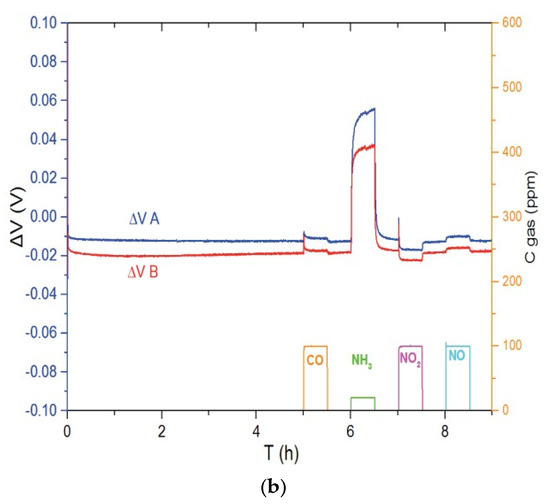
Figure 3.
Response of the (a) Ni3V2O8 sensors at 700 °C and (b) Au-V2O5 sensors at 600 °C for pulses of CO (100 ppm), NH3 (20 ppm), NO2 (100 ppm) and NO (100 ppm) in base gas.
The operating temperature of 700 °C was chosen for Ni3V2O8 based sensor because no significant response was monitored for weaker temperature. In order to study the influence of temperature on selectivity and to emphasize the interest of V2O5 based electrodes, responses of Pt/YSZ/Au-V2O5 sensors are compared in Table 1 to the ones of a Pt/YSZ/Au sensor studied in a previous study [8]. By comparing these results, we can observe that the responses related to Au gas sensors decrease considerably by increasing temperature. However, regarding Au-V2O5 sensors, the cross sensitivity to other interfering gases can be completely eliminated at 600 °C and selective ammonia sensor is achieved.

Table 1.
Comparison of Au and Au-V2O5 sensor responses (∆V in mV) at different temperatures to pulses of CO (100 ppm), NH3 (20 ppm), NO2 (100 ppm) and NO (100 ppm) in base gas (∆V experimental uncertainties around 5 mV).
4. Conclusions
Two vanadium-based working electrodes were tested in order to find a sensitive ammonia sensor at high temperature. By comparing all these results, we can conclude that although both Ni3V2O8 and Au-V2O5 sensors are sensitive to ammonia, Au-V2O5 sensors show better sensitivity and selectivity toward NH3 at operating temperature of 600 °C.
Further characterizations of electrode materials is yet to be done in order to better understand the specific interaction with ammonia, leading to higher selectivity.
References
- ASuarez-Bertoa, R.; Zardini, A.A. Ammonia exhaust emissions from spark ignition vehicles over the New European Driving Cycle. Atmosp. Environ. 2014, 97, 43–53. [Google Scholar] [CrossRef]
- Daia, L.; Yanga, G.; Zhou, H.; He, Z.; Li, Y.; Wang, L. Mixed potential NH3 sensor based on Mg-doped lanthanum silicate oxyapatite. Sens. Actuators B 2016, 224, 356–363. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. Solid state potentiometric gas sensors-current status and future trends. J. Solid State Electr. J. 2009, 13, 3–25. [Google Scholar] [CrossRef]
- Meng, W.; Dai, L.; Meng, W.; Zhou, H.; Li, Y.; He, Z.; Wang, L. Mixed-potential type NH3 sensor based on TiO2 sensing electrode with a phase transformation effect. Sens. Actuators B 2017, 240, 962–970. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Xia, F.; Zhang, H.; Xiao, J. Effect of V2O5-content on electrode catalytic layer morphology and mixed potential ammonia sensor performance. Sens. Actuators B 2016, 223, 658–663. [Google Scholar] [CrossRef]
- Modafferi, V.; Trocino, S.; Donato, A.; Panzera, G.; Neri, G. Electrospun V2O5 composite fibers: Synthesis, characterization and ammonia sensing properties. Thin Solid Films 2013, 548, 689–694. [Google Scholar] [CrossRef]
- Liu, F.; Sun, R.; Guan, Y.; Cheng, X.; Zhang, H.; Guan, Y.; Liang, X.; Sun, P.; Lu, G. Mixed-potential type NH3 sensor based on stabilized zirconia and Ni3V2O8 working electrode. Sens. Actuators B 2015, 210, 795–802. [Google Scholar] [CrossRef]
- Nematbakhsh Abkenar, G.; Viricelle, J.P.; Breuil, P. Study of YSZ Electrolyte Inks for Preparation of Screen-Printed Mixed-Potential Sensors for Selective Detection of NOx and NH3. In Proceedings of the Eurosensors 2017, Paris, France, 3–6 September 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).