Enzymatic Sensor Based on Dye Sensitized TiO2 Electrode for Detection of Catechol in Water †
1. Summary
2. Materials and Methods
2.1. Cell Construction
2.2. Nanoporous-TiO2 Film Sintering
2.3. Sensitation with Dye Iron Phthalocyanine (FePc), Nafion and Enzyme Laccase from Trametes Versicolor
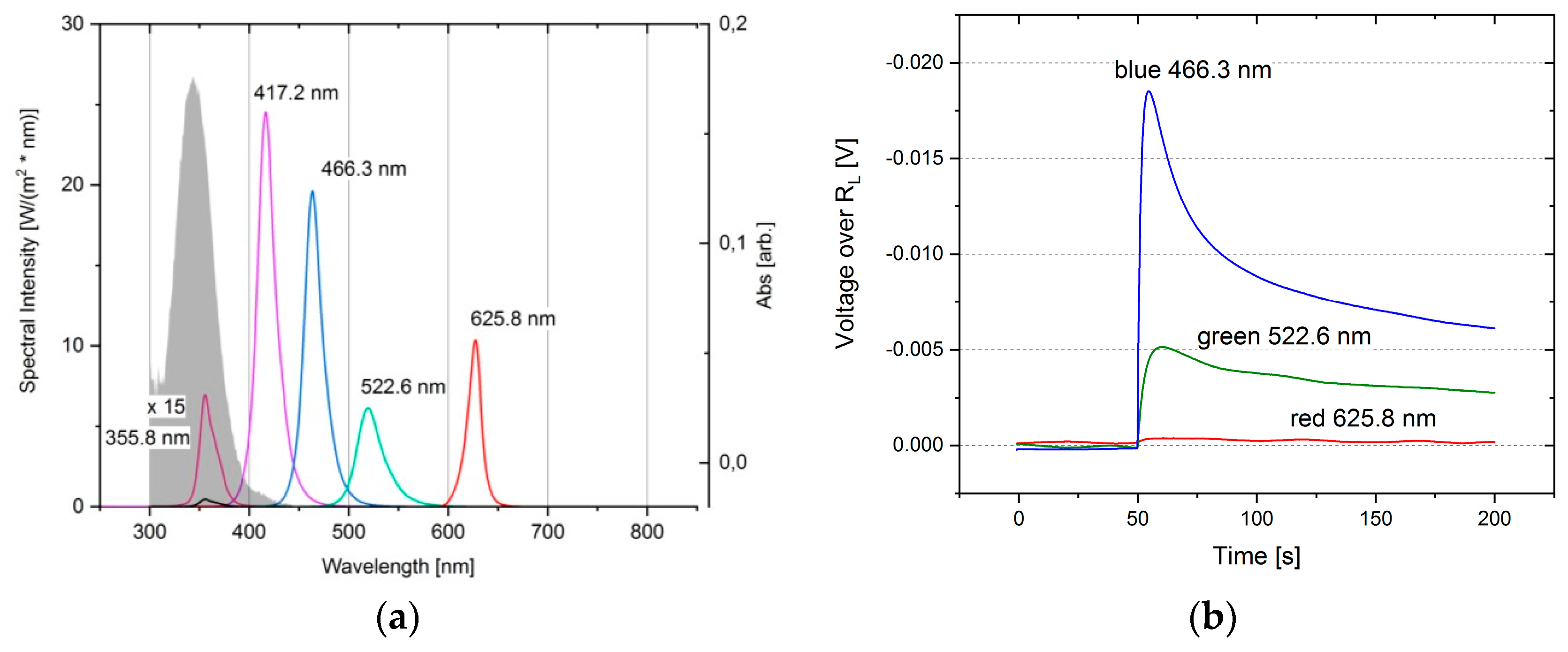
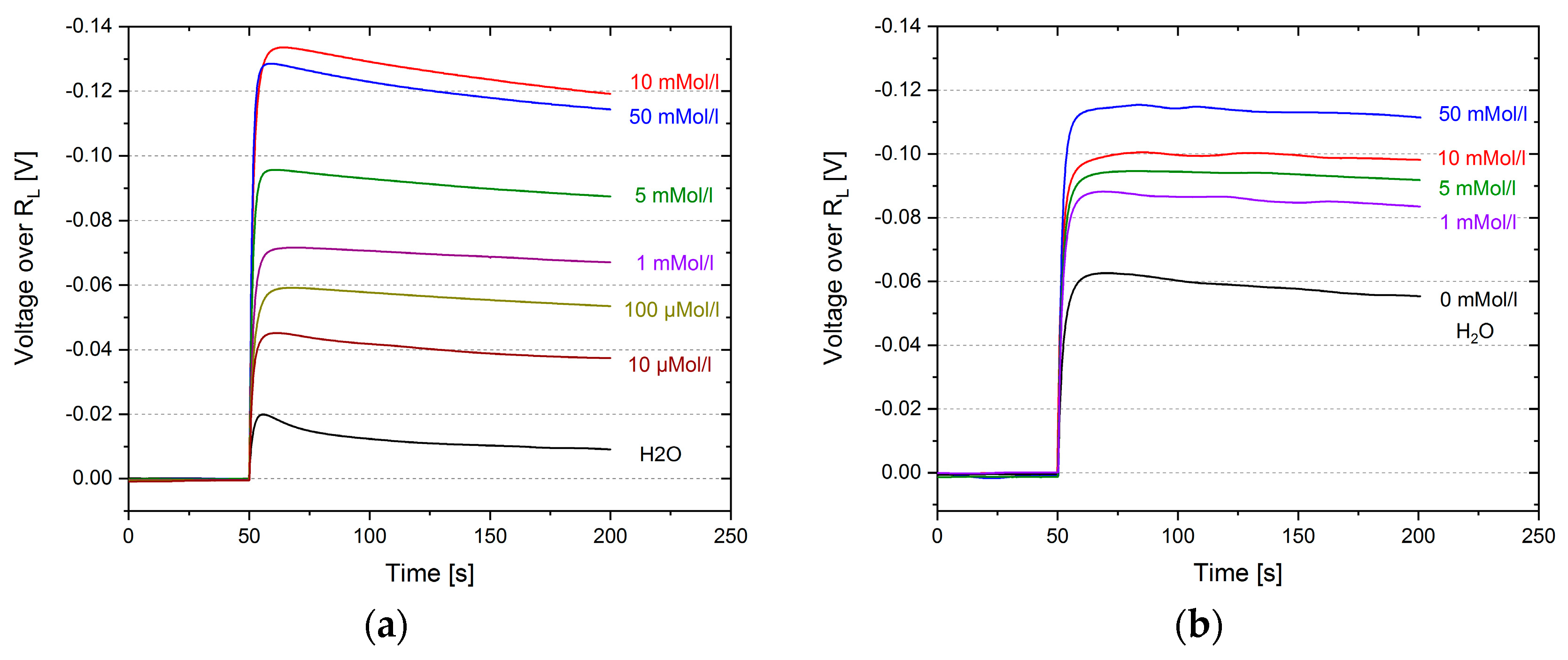
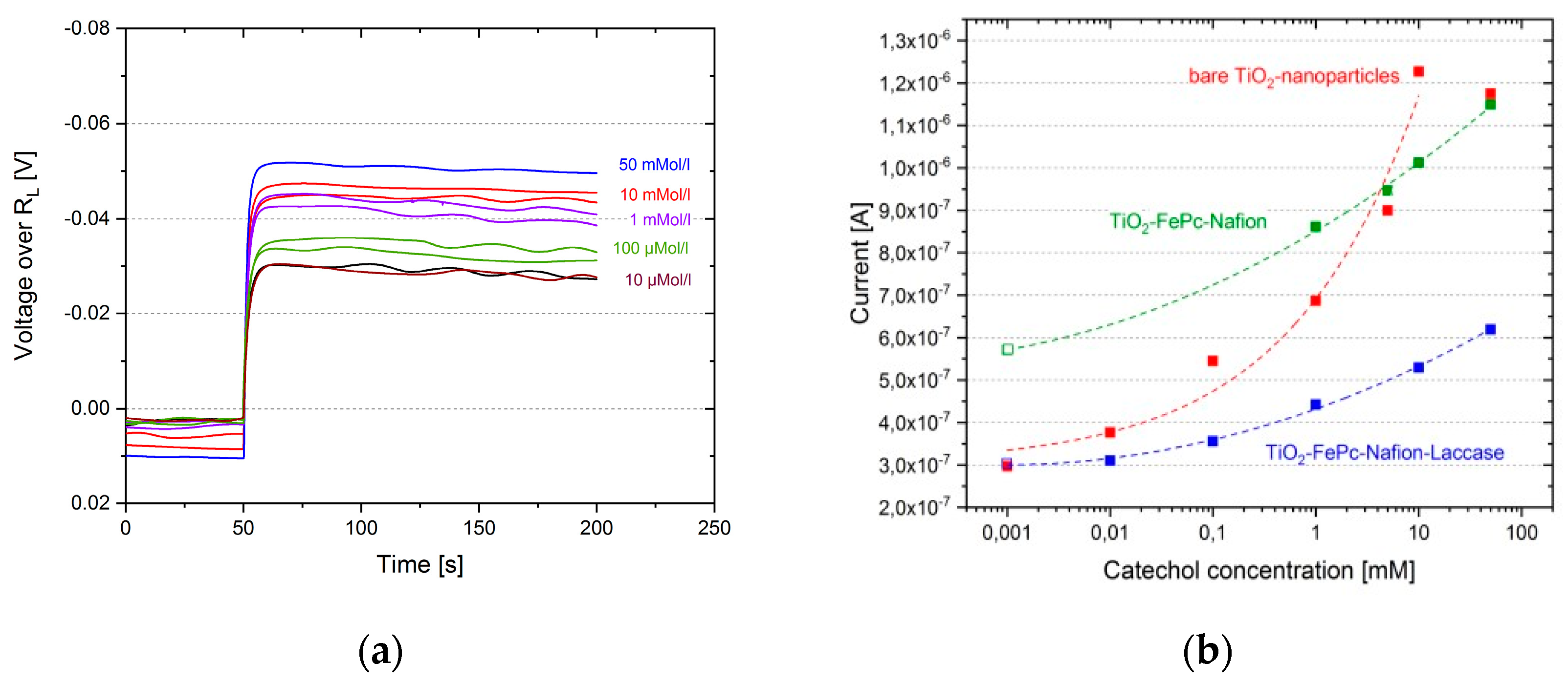
3. Results
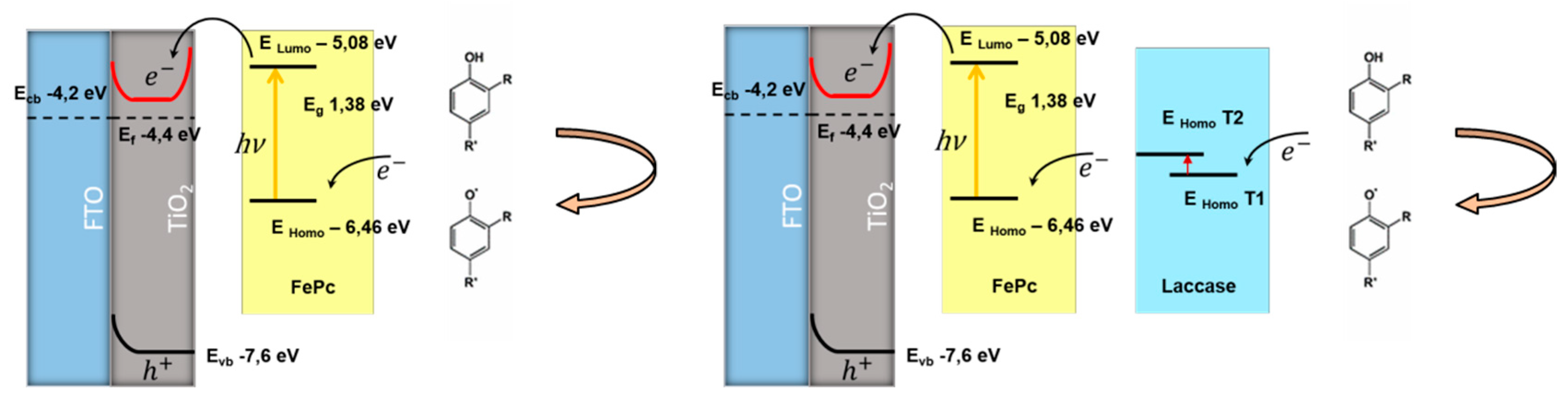
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rajeshwar, K. Fundamentals of Semiconductor Electrochemistry and Photoelectrochemistry, Encyclopedia of Electrochemistry; Wiley-VCH Verlag GmbH &, Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Siris, R.; Boudaden, J.; Klumpp, A. Photoelectrochemical nitrate sensor utilizing Cu/Pd nanoparticles on TiO2-nanoparticles carrier. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Sosna, M.; Chrétien, J.M.; Kilburn, J.D.; Bartlett, P.N. Monolayer anthracene and anthraquinone modified electrodes as platforms for Trametes hirsuta laccase immobilization. Phys. Chem. Chem. Phys. 2010, 12, 10018–10026. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klumpp, A.; Adrovic, A.; Boudaden, J. Enzymatic Sensor Based on Dye Sensitized TiO2 Electrode for Detection of Catechol in Water. Proceedings 2018, 2, 737. https://doi.org/10.3390/proceedings2130737
Klumpp A, Adrovic A, Boudaden J. Enzymatic Sensor Based on Dye Sensitized TiO2 Electrode for Detection of Catechol in Water. Proceedings. 2018; 2(13):737. https://doi.org/10.3390/proceedings2130737
Chicago/Turabian StyleKlumpp, Armin, Asmir Adrovic, and Jamila Boudaden. 2018. "Enzymatic Sensor Based on Dye Sensitized TiO2 Electrode for Detection of Catechol in Water" Proceedings 2, no. 13: 737. https://doi.org/10.3390/proceedings2130737
APA StyleKlumpp, A., Adrovic, A., & Boudaden, J. (2018). Enzymatic Sensor Based on Dye Sensitized TiO2 Electrode for Detection of Catechol in Water. Proceedings, 2(13), 737. https://doi.org/10.3390/proceedings2130737





